3887
Brain mechanisms underlying Gulf War Illness revealed by connectomics signatures of the disease1Department of Radiology & Imaging Sciences, Emory University, Atlanta, GA, United States, 2University of Houston-Clear Lake, Houston, TX, United States, 3Department of Neurology, Emory University, Atlanta, GA, United States, 4Atlanta VA Medical Center, Decatur, GA, United States, 5Department of Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Around 200,000 veterans (up to 32% of those deployed) of the 1991 Gulf War (GW) suffer from GW illness (GWI), which is characterized by multiple deficits in cognitive, emotion, sensory and nociception domains. In this study we examined 60 GWI patients and 30 age-matched controls with resting state fMRI (rsFMRI) in order to map impairments in brain function networks in GWI with group independent components analysis. GWI veterans exhibited impaired/abnormally increased FC between different brain function networks, revealing brain mechanisms underlying neurological impairments in GWI.
INTRODUCTION
Around 200,000 veterans (up to 32% of those deployed) of the 1991 Gulf War (GW) suffer from GW illness (GWI). GWI is a poorly understood chronic medical condition, characterized by multiple symptoms indicative of brain function deficits in cognitive, affective, sensory and nociception domains1-6. In this study, we employed resting state fMRI (rsFMRI) to explore impairments in brain function networks in GWI, with group independent components analysis7 (GICA).METHODS
60 GWI veterans (mean age 49.4 yrs.) and thirty healthy veteran controls (VC) (mean age 49.8 yrs.), were scanned in a Siemens 3T MRI scanner using a 12-channel Rx head coil. Written informed consent was obtained from all participants in the protocol approved by the local Institutional Review Board. rsFMRI data were acquired with a 10-min whole-brain gradient echo EPI (TR/TE/FA = 2000/24ms/90°, resolution = 3mm x 3mm x 3.5mm). rsFMRI preprocessing steps included attenuation of signal related to subject-motion and physiological responses, using the AROMA techniques8, and spatial smoothing with FWHM = 6mm isotropic Gaussian kernel. A group spatial ICA7 was performed on temporally concatenated data of the whole group of 90 subjects, with the Infomax algorithm, using the GIFT software9. For each subject, the functional connectivities (FCs) between the different IC networks (obtained through back-projection7 of the group ICs), were assessed with cross-correlation (CC) analysis. Between-group differences in FCs were assessed with 2-sample t-tests on z-transformed CCs, corrected for multiple comparisons through an FDR method10.RESULTS & DISCUSSION
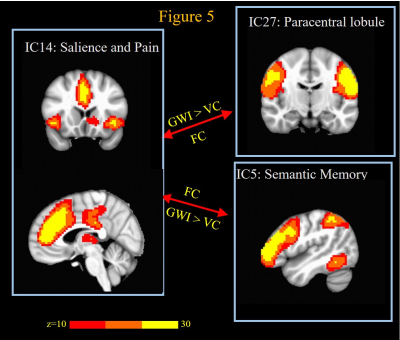
GWI veterans exhibited significantly (FDR q < 0.05) decreased FCs compared to VC (Fig. 1-2) between motor networks and sensory perception networks of different modalities (somatosensory, visual and auditory). This is consistent with their difficulties in performing complex visuomotor, audiomotor and sensorimotor tasks1,2,6. GWI veterans exhibit impaired FC between sensory perception networks of different modalities (e.g. auditory and visual); as well as impaired FC between multisensory perception networks and motor and cognitive centers. This indicates problems with multisensory integration and could explain symptoms of confusion and ataxia observed in GWI1,11. GWI veterans exhibited impaired FC between language (e.g., word-generation and semantic memory) networks, and both sensory input and motor output networks, which could explain their difficulties with tasks like word-finding1,2,5,6. GWI also exhibited (Fig 3-4) impaired cerebellar FC with all sensory perception and motor networks, which could be related to their sensory and vestibular deficits described above. GWI veterans also exhibited impaired cerebellar FC to language networks as well as medial and lateral prefrontal cortex regions which are involved in a number of cognitive functions12,13. Thus, it is possible that cognitive difficulties seen in GWI could also be caused by these impaired cerebello-cognitive connections. Thus, cerebellum seems to be at the center of brain function network impairments in GWI. Cerebellum works in concert with basal ganglia and cortex in the execution of almost all brain functions12,13. Epidemiologic and animal studies have associated GWI with exposure to neurotoxic chemicals such as nerve agents and organophosphate pesticides (OP), all of which are known to impair the cholinergic system1,11,14. Cerebellum is richly innervated by cholinergic projections15, and has been found to be impaired in GWI veterans’ studies from other research groups too16,17. Cerebellum is also implicated by animal models of sarin and organophosphate exposures14. Thus cerebellar impairment from neurotoxic exposures is one putative mechanism for GWI. On the other hand, GWI veterans exhibited abnormally increased FC between different pain processing related ICs. This is consistent with chronic pain symptoms of GWI1,4,11. Further, ill GW veterans also exhibited ICs abnormally increased FC between language network, and salience and pain processing network. This could be due to increased access of salience function resources by language centers to compensate for decreased or confusing signals from stimulus perception regions. This could also reflect recruitment of brain areas involved in language into an overactive pain neuromatrix18 thereby interfering with language function.
Acknowledgements
This work was supported by the Office of Assistant Secretary of Defense for Health Affairs, through the Gulf War Illness Research Program under Award No.W81XWH-16-1-0744 (PI: Gopinath). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.References
1. Binns JH, Barlow C, Bloom FE, Clauw DJ, Golomb BA, Graves JC. Report of Research Advisory Committee on Gulf War Veterans’ Illnesses. In: Affairs DoV, ed. Boston, MA: U.S. Government Printing Office; 2014.
2. Hom J, Haley RW, Kurt TL. Neuropsychological correlates of Gulf War syndrome. Arch Clin Neuropsychol 1997;12:531-44.
3. Calley CS, Kraut MA, Spence JS, Briggs RW, Haley RW, Hart J, Jr. The neuroanatomic correlates of semantic memory deficits in patients with Gulf War illnesses: a pilot study. Brain Imaging Behav 2010;4:248-55.
4. Gopinath K, Gandhi P, Goyal A, et al. FMRI reveals abnormal central processing of sensory and pain stimuli in ill Gulf War veterans. Neurotoxicology 2012;33:261-71.
5. Moffett K, Crosson B, Spence JS, et al. Word-finding impairment in veterans of the 1991 Persian Gulf War. Brain Cogn 2015;98:65-73.
6. Toomey R, Alpern R, Vasterling JJ, et al. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J Int Neuropsychol Soc 2009;15:717-29.
7. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 2001;14:140-51.
8. Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 2015;112:267-77.
9. Group ICA of fMRI Toolbox (GIFT). at http://mialab.mrn.org/software/gift/.)
10. Soric B. Statistical “Discoveries” and Effect-Size Estimation. Journal of American Statistical Association 1989;84:608-10.
11. Haley RW, Kurt TL, Hom J. Is there a Gulf War Syndrome? Searching for syndromes by factor analysis of symptoms. JAMA 1997;277:215-22.
12. Baumann O, Borra RJ, Bower JM, et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum 2015;14:197-220.
13. Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 2018;19:338-50.
14. Bhardwaj S, Musalgaonkar N, Waghmare C, Bhattacharya BK. Single dose exposure of sarin and physostigmine differentially regulates expression of choline acetyltransferase and vesicular acetylcholine transporter in rat brain. Chem Biol Interact 2012;198:57-64.
15. Zhang C, Zhou P, Yuan T. The cholinergic system in the cerebellum: from structure to function. Rev Neurosci 2016;27:769-76.
16. Christova P, James LM, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP. Subcortical brain atrophy in Gulf War Illness. Exp Brain Res 2017.
17. Rayhan RU, Stevens BW, Raksit MP, et al. Exercise challenge in Gulf War Illness reveals two subgroups with altered brain structure and function. PLoS One 2013;8:e63903.
18. Nijs J, Meeus M, Van Oosterwijck J, et al. In the mind or in the brain? Scientific evidence for central sensitisation in chronic fatigue syndrome. Eur J Clin Invest 2012;42:203-12.
Figures