3754
Long-term effect of isoflurane anesthesia on functional connectivity detected by fMRI and local field potential measurements1A.I. Virtanen Institute for Molecular Sciences, Kuopio, Finland
Synopsis
Isoflurane, a commonly used anesthetic in preclinical studies, is known to alter functional connectivity during anesthesia. It has been found that isoflurane can induce brain plasticity and cause long-term changes on brain function. Therefore, we studied the connectivity changes caused by single isoflurane (3h, 1.8%) exposure after a one-month waiting period by resting state fMRI and local field potential (LFP) measurements. Treated rats exhibited significantly strengthened connectivity between hippocampus and somatosensory cortex both in fMRI and LFP, indicating long-term modulation of brain activity by single administration of isoflurane anesthesia compared to non-treated controls.
Introduction
In preclinical fMRI studies, anesthesia is typically required to minimize stress and movement of the animals during the measurements. Anesthesia can, however, dramatically alter brain function1 and/or change hemodynamic response2,3. Furthermore, it has been shown that the effects of anesthetics can extend beyond the duration of anesthesia. For example, isoflurane can influence plasticity and gene-expression several days or weeks after the initial exposure.4-12 Several experiments include, e.g., long surgical procedures13 under general anesthesia, that can possibly have a long-term effect on brain connectivity14, 15. However, no studies have been conducted to evaluate the long-term effects of isoflurane anesthesia on functional connectivity. Therefore, the aim of the study was to investigate the long-term effect of 1.8% isoflurane on brain function using fMRI and electrophysiological local field potential (LFP) measurements a month after the initial exposure.Subjects and methods
Male Wistar rats (n = 12) were exposed to 1.8%
isoflurane for 3 h and naïve rats (n=12) were used as controls. This protocol
was mimicking the anesthesia during a long invasive surgical procedure. After
one month, 6+6 rats were imaged with fMRI and another group of 6+6 rats were
used in LFP measurements. FMRI and LFP measurements were conducted with the
following isoflurane concentrations: 1.3%, 2.0%, 1.3%, and 3.0%, each 10min in
duration. FMRI was performed in a Bruker Pharmascan 7T magnet with single-shot
spin-echo echo planar imaging sequence with the following parameters: TR 2,000
ms, TE 45 ms, matrix size 64 × 64, field-of-view 2.5 × 2.5 cm, 11 slices of 1.5
mm thickness, and a bandwidth of 250 kHz. FMRI data was converted to NIfTI (http://aedes.uef.fi),
slice-timing corrected, motion-corrected, co-registered to a reference brain
(SPM8), and finally smoothed. Resting state functional connectivity (RSFC) (0.01 - 0.15 Hz) was calculated from either 4 or 12 regions of interest, covering the
LFP electrode locations or whole brain, respectively.
LFP was recorded with SciWork data acquisition
system (Datawave Technologies) with a 2049 Hz sampling rate. Cortical LFP was
recorded bilaterally from the somatosensory cortex (S1) left (S1L) and right
(S1R) (AP:-1, ML: +/- 3) using screw electrodes, and hippocampal LFP (50µm stainless steel wire electrodes) from the right dentate gyrus (DG)
(AP: -3.8, ML: + 1.6, DV: -4.3) and right cornu ammonis 1 (CA1) (AP: -3.8, ML:
+ 1.6, DV: -3.7). Data was analyzed with Matlab R2011a and Spike2, version 8. LFP
coherence was analyzed from averaging 30s long epochs during each isoflurane
concentration. Inter-channel correlation was measured from LFP power (amplitude
envelope) using either full band or band-pass filtered signal.16,17 Burst
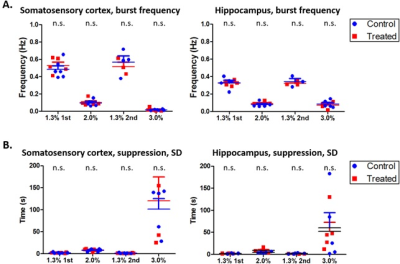
suppression activity of burst frequency (Hz) and standard deviation (SD) of
suppression periods (s) was also analyzed.
Statistical testing for fMRI correlation, LFP coherence and correlation were conducted by two-tailed two-sample t-tests in Matlab. We used false discovery rate (FDR) to account for multiple comparisons for 12-ROI correlation and LFP coherence analyzes.
Results
Both fMRI and LFP measurements indicated that brain function was altered one month after the initial isoflurane exposure. In fMRI whole brain analysis, we found increased correlation in 11 thalamo-cortical and in 5 hippocampal-cortical connections in the isoflurane-exposed group compared to the control group (p<0.05, FDR adjusted) under 2.0% isoflurane (Figure 1). Also, increased CA1-S1R correlation was found in the 4-ROI fMRI analysis (p=0.025) under 2.0% isoflurane (Figure 2).
In LFP analysis, we found increased coherence between DG and S1R in delta band during the 2nd 1.3% isoflurane period (p=0.026, FDR adjusted) (Figure 3). Additionally, increased coherence was observed between DG and S1L in alpha band during the 1st 1.3% isoflurane period (p=0.034, FDR adjusted) and in delta band under the 2nd 1.3% isoflurane period (p=0.009, FDR adjusted). Correlation of LFP power was increased between S1R and DG, and S1R and CA1 (p=0.035 and 0.044 accordingly) in isoflurane exposed rats compared to controls under 1.3% isoflurane (Figure 2). Finally, no change in burst frequency or SD of suppression periods was found in either somatosensory cortex (p=0.470 and p=0.676, respectively) or in hippocampus (p=0.377 and p=0.676) (Figure 4).
Discussion and conclusion
These results suggest that a single prolonged isoflurane exposure has a persistent effect on brain function that lasts at least one month. Because no change was seen in burst frequency (established measures for the depth of anesthesia) or SD of suppression periods, the observed alterations in FC likely reflect neural network plasticity changes rather than a change in the depth of anesthesia. As a conclusion, in studies exploiting deep isoflurane anesthesia, extra caution should be taken while interpreting connectivity results, even if there is a long period after initial anesthesia.Acknowledgements
This work was supported by the Doctoral Program in Molecular Medicine of University of Eastern Finland. We thank MP for technical assistance in animal preparations. The funding sources had no further role in study design, or in the collection, analysis or interpretation of data.References
1. Paasonen J, Stenroos P, Salo RA, Kiviniemi V, Gröhn O. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. Neuroimage. 2018 May 15;172:9-20. doi: 10.1016/j.neuroimage.2018.01.014.
2. Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural–hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006 Aug 1;32(1):33-48.
3. Ogawa S, Lee TM, Kay AR and Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci 1990;87:9868-9872.
4. Long Ii RP, Aroniadou-Anderjaska V, Prager EM, Pidoplichko VI, Figueiredo TH, Braga MF, 2016. Repeated Isoflurane Exposures Impair Long-Term Potentiation and Increase Basal GABAergic Activity in the Basolateral Amygdala. Neural Plast. 2016, 8524560.
5. Uchimoto K, Miyazaki T, Kamiya Y, Mihara T, Koyama Y, Taguri M, Inagawa G, Takahashi T, Goto T, 2014. Isoflurane impairs learning and hippocampal long-term potentiation via the saturation of synaptic plasticity. Anesthesiology 121, 302–10.
6. Joksovic PM, Lunardi N, Jevtovic-Todorovic V, Todorovic SM. Early exposure to general anesthesia with isoflurane downregulates inhibitory synaptic neurotransmission in the rat thalamus. Mol Neurobiol. 2015 Oct;52(2):952-8. doi: 10.1007/s12035-015-9247-6.
7. Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgänsberger W, Ohl F, Kochs EF, Blobner M. Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009 Mar;56(3):626-36. doi: 10.1016/j.neuropharm.2008.11.002.
8. Culley DJ, Yukhananov RY, Xie Z, Gali RR, Tanzi RE, Crosby G. Altered hippocampal gene expression 2 days after general anesthesia in rats. Eur J Pharmacol. 2006 Nov 7;549(1-3):71-8.
9. Zhong T, Guo Q, Zou W, Zhu X, Song Z, Sun B, He X, Yang Y. Neonatal Isoflurane Exposure Induces Neurocognitive Impairment and Abnormal Hippocampal Histone Acetylation in Mice. PLoS One. 2015 Apr 30;10(4):e0125815. doi: 10.1371/journal.pone.0125815.
10. Colon E, Bittner EA, Kussman B, McCann ME, Soriano S, Borsook D. Anesthesia, brain changes, and behavior: Insights from neural systems biology. Progress in neurobiology. 2017, DOI: 10.1016/j.pneurobio.2017.01.005.
11. Uchimoto K, Miyazaki T, Kamiya Y, Mihara T, Koyama Y, Taguri M, Inagawa G, Takahashi T, Goto T, 2014. Isoflurane impairs learning and hippocampal long-term potentiation via the saturation of synaptic plasticity. Anesthesiology 121, 302–10.
12. Joksovic PM, Lunardi N, Jevtovic-Todorovic V, Todorovic SM. Early exposure to general anesthesia with isoflurane downregulates inhibitory synaptic neurotransmission in the rat thalamus. Mol Neurobiol. 2015 Oct;52(2):952-8. doi: 10.1007/s12035-015-9247-6.
13. Rundshagen I. Postoperative Cognitive Dysfunction. Dtsch Arztebl Int. 2014 Feb; 111(8): 119–125. doi: 10.3238/arztebl.2014.0119.
14. Xie P, Yu T, Fu X, Tu Y, Zou Y, Lui S, Zhao X, Huang X, Kemp GJ, Gong Q. Altered Functional Connectivity in an Aged Rat Model of Postoperative Cognitive Dysfunction: A Study Using Resting-State Functional MRI. PLoS One. 2013; 8(5): e64820., May 30. doi: 10.1371/journal.pone.0064820
15. Taghon TA, Masunga AN, Small RH, Kashou NH. A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Paediatr Anaesth. 2015 Mar;25(3):239-46. doi: 10.1111/pan.12606.
16. Liu X, Zhu XH, Zhang Y, Chen W. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb Cortex. 2011 Feb;21(2):374-84. doi: 10.1093/cercor/bhq105.
17. Liu X, Zhu XH, Zhang Y, Chen W. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr. 2013 Jul;26(3):363-77. doi: 10.1007/s10548-012-0267-5.
Figures