3620
Assessment of Microstructural Changes Induced via Repeated Videogame Training as a Measure of Neuroplasticity in Normal Developing, College-age Brains1University of Wisconsin-Madison, Madison, WI, United States, 2Indiana University-Bloomington, Bloomington, IN, United States
Synopsis
In this study, diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI) were used to assess brain changes in white matter regions as a result of repeated videogame training. In a cohort playing a simulated race car game, we observe evidence of microstructural changes in tracts associated with working and long-term memory. Subjects playing a guitar simulation game, experienced changes in regions associated with attention, visuomotor learning, and proprioception of the limbs. This study demonstrates that diffusion MRI is promising for characterizing subtle brain changes associated with learning.
Introduction
Neuroplasticity refers to the ability of the brain to adapt and change in response to experiential and environmental factors1. Recent studies have reinforced the emerging view that the brain retains significant plasticity throughout the lifespan and have shown that a variety of forms of behavioral training can induce clear neuroplastic changes2,3,4. Diffusion weighted imaging (DWI) has been shown to capture such plasticity-related changes within white matter (WM). Diffusion tensor imaging (DTI) and the more recent neurite orientation dispersion and density imaging (NODDI)5 are able to quantify measures of WM microstructure, namely fractional anisotropy (FA), mean diffusivity (MD), orientation dispersion index (ODI), and intracellular volume fraction (FICVF), that can be used to assess variations within WM. One form of behavioral training that has garnered a great deal of scientific interest with regard for its potential to alter various core human abilities is training with commercial video games6. In this work, we discuss the neuroplastic changes observed as a result of long-term videogame training in a cohort of normally-developing, college-age participants.Methods
Sixty college-age participants were divided into 3 groups: two video-game training groups - Need-for-Speed (NFS; n=20; 6M, 14F) and Guitar Hero (GH; n = 20; 5M, 15F) - and a Control (n=20; 5M, 15 F) group. The two training video games were hypothesized to cause changes in different brain networks i.e., spatial working memory in NFS, motor learning in GH. Participants received MRI scans on three occasions (T1) baseline, (T2) after 90 minutes of game training, and (T3) after 10 sessions of 90 minutes of game training over ~3-4 weeks. Here we report the changes between T1 and T3. Each training session lasted 90 minutes. The NFS cohort played the Need for Speed: Shift7 car racing game and completed as many laps of the same race course as possible in each session. The track used was changed halfway through the 3-6 week training period. This NFS game was used in a prior study of short-term brain plasticity8. The GH cohort played the Guitar Hero9 simulated guitar playing game for roughly 90 minutes in each session. The bulk of the songs were neither repeated within session nor across sessions; however, one standard song was repeated for each session to estimate changes in performance. Participants were scanned at 3T using a 32-channel head RF coil. Multi-b DWI was performed (b=0, 350, 800, and 2000s/mm2) with 69 encoding directions at 2mm isotropic resolution. Data were processed using an in-house processing pipeline utilizing DIPY, FSL10, and MRtrix11. Processing included corrections for Rician noise12, Gibbs ringing13, eddy-currents14,15, and susceptibility distortions. Diffusion tensors were calculated using WLS fitting DIPY and NODDI measures using AMICO16.Within-subject co-registration followed by between-subject spatial normalization was performed using ANTs17. DTI and NODDI maps were transformed into this space. The JHU White Matter atlas18 was used to extract 18 ROIs. Means were calculated for each DTI and NODDI measure and paired t-tests were computed comparing long term (T1 to T3) data within groups. Statistical testing with Bonferroni multiple comparisons correction (factor of 18) was used.Results
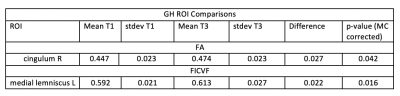
Table 1 shows significant results in the NFS cohort, particularly bilateral decreases in MD in the hippocampal portion of the cingulum, as well as decreases in RD and AD in the right and left hippocampal portions of the cingulum, respectively. Further, we observed NODDI index increases within the right hippocampal portion of the cingulum and the right anterior limb of the internal capsule. Table 2 shows significant increases in GH of FA and FICVF in the right cingulum and the left medial lemniscus, respectively.No significant results were observed for controls.Discussion
In NFS, we observed microstructural changes in the hippocampal cingulum bundles, which provide connections to the hippocampal and peri-hippocampal gyrus areas (which showed changes following short-term training with NFS ) and have been associated with spatial learning and working memory18.In the GH cohort, FA and FICVF increases were observed for a visuomotor learning task. The peri-callosal cingulum tracts are linked to visuomotor learning, motor planning, attention and working memory19. The medial lemniscus carries proprioceptive and sensory-motor afferents from the limbs and fingers20; which may be related to learning the intricate positioning and depression of digits needed to play GH.Acknowledgements
No acknowledgement found.References
1, 2,8,19Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the Fast Lane: New Insights into Neuroplasticity. Neuron 73:1195–1203
3Keller TA, Just MA. Structural and functional neuroplasticity in human learning of spatial routes. Neuroimage 2016;125:256–266.
4Taubert, M, Lohmann, G, Margulies, DS, Villringer, A, Ragert, P, 2011. Long-term effects of motor training on resting-state networks and underlying brain structure. NeuroImage 57, 1492-1498.
5Zhang, H., Schneider, T., Wheeler-Kingshott, C. A., & Alexander, D. C. (2012). NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 61(4), 1000-1016. 6Meta-Analysis of Action Video Game Impact on Perceptual, Attentional, and Cognitive SkillsBediou, B.,Adams, D.,Mayer, R.,Tipton, E.,Green, C.S.,Bavelier, D.(2017)Psychological Bulletin.https://dx.doi.org/10.1037/bul0000130
7Electronic Arts (2009), Need for Speed: Shift, video game, Xbox 360, Redwood City, California.
9Activision (2009), Guitar Hero, video game, Xbox 360, Santa Monica, California.
10M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62:782-90, 2012
11Tournier, J. D., Calamante, F., & Connelly, A. (2012). MRtrix: diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology, 22(1), 53-66.
12Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394-406.
13Kellner, E; Dhital, B; Kiselev, V.G & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine, 2016, 76, 1574–1581.
14Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-78.
15Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556-72
16Daducci, A., Canales-Rodríguez, E. J., Zhang, H., Dyrby, T. B., Alexander, D. C., & Thiran, J. P. (2015). Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. NeuroImage, 105, 32-44.
17Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 54(3), 2033-2044.
18Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PC, Mazziotta J, Mori S. Neuroimage. 2008 Nov 15;43(3):447-57.
20Takahashi, M., Iwamoto, K., Fukatsu, H., Naganawa, S., Iidaka, T., & Ozaki, N. (2010). White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neuroscience letters, 477(2), 72-76. 22Cramer, G., Darby, S. Clinical Anatomy of the Spine, Spinal Cord, and Ans (Third Edition)2014, Pages 341-412
Figures