3500
Optimizing Diffusion MRI for Mapping Cortical Fiber Patterns1Stanford University, Stanford, CA, United States
Synopsis
A custom DW pulse sequence is optimized to minimize echo time and evaluate optimal trade-offs between SNR, b-value and spatial resolution for mapping cortical fiber patterns. Using a lower b-value (500 vs. 1000 s/mm2) failed to improve estimates of the principal fiber orientation in the cortex despite the significant boost in signal. Accurate estimates of the principal fiber orientation in the cortex requires a spatial resolution of 1.44 mm isotropic or less.
Introduction
Mapping cortical fiber patterns using DWI has significant clinical relevance in the assessment of many disease processes including epilepsy1, Alzheimer's2 and multiple sclerosis3, which each have been shown to have significant cortical involvement. Conventional DTI has seen many optimizations for the assessment of white matter but much less is understood regarding the optimal sequence design and protocol for mapping cortical fiber patterns. Given that cortical axons and dendrites are significantly less dense compared to white matter and capturing only the dominant orientation within cortex may be sufficient for significant clinical relevance, the optimal encoding strategy is less clear. Here, a custom DW pulse sequence is optimized to minimize echo time and evaluate optimal trade-offs between SNR, b-value and spatial resolution for mapping cortical fiber patterns.Methods
Data Acquisition:
In accordance with IRB approval two 1.5 hr scans were conducted on the same healthy subject using a 3T whole-body MR system (Premier, GE Healthcare, Madison, Wisconsin) equipped with a 48-channel phased array head coil (GE Healthcare). The subject was examined with a custom DW pulse sequence with the second diffusion gradient pulse split across the refocusing pulse to reduce the echo time (Fig. 1). Coronal DW images were acquired over a field-of-view covering the motor and somatosensory cortex at b=500 and 1000 s/mm2. The scan parameters are shown in Table 1. For each b-value, 45 b0 images and 900 diffusion directions uniformly distributed over a sphere were acquired. An additional 9 b0 images were acquired with the phase encoding polarity reversed for EPI distortion correction. T1w memprage4 structural images were also acquired for each scan session.
Data Analysis:
Diffusion images were corrected for eddy current distortions and bulk motion using “eddy” and “topup” from the FMRIB Software Library5,6. Random subsets of 100-900 of the diffusion directions were used to fit the diffusion tensor. The transformation between the diffusion space and the structural image space was found by co-registering the fractional anisotropy map derived from the full dataset to the memprage image. The angular similarity between the primary orientations estimated using the sub-sampled datasets and the full dataset was used to evaluate the effect of SNR and angular resolution on the estimates of the primary diffusion orientation within regions of interest derived from the memprage images using FreeSurfer7. The effect of spatial resolution on estimates of the principal diffusion orientation within the cortex was also evaluated by smoothing the diffusion images by setting the outer regions of k-space to 0. The diffusion tensor was fit to data smoothed to 2, 4, 6, 8, and 27 mm3 isotropic voxels and the angular similarity of the principal orientation was compared to the original 1 mm3 isotropic data.
Results
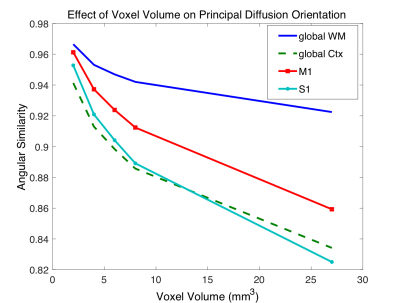
Figure 2 shows the estimated primary fiber orientation in the motor and somatosensory cortex as a function of the number of diffusion directions for both b=500 and 1000 s/mm2. Figure 3 shows the angular similarity (1 is aligned) between the primary fiber orientation estimated from various levels of down-sampled diffusion data at b=500 and 1000 s/mm2. Figure 4 shows the angular similarity between primary orientations estimated using the original 1 mm data and data downsampled to a coarser resolution.Discussion
As shown in Figures 2 and 3, the diffusion orientation estimates from the data subsets acquired at b = 1000 s/mm2 are more closely aligned with the estimates from the full dataset over a larger range of diffusion directions compared to b = 500 s/mm2. This results suggests that a minimal diffusion contrast of b=1000 s/mm2 is critical for estimating dominant cortical orientations. Figure 4 suggests that accurate estimates of the primary fiber orientation in the cortex can be obtained by voxels up to 2-3 mm3 in total volume (i.e. 1.26 to 1.44 mm isotropic dimensions).Conclusion
Using a lower b-value (500 vs. 1000 s/mm2) failed to improve estimates of the principal fiber orientation in the cortex despite the significant boost in signal. Accurate estimates of the principal fiber orientation in the cortex requires a spatial resolution of 1.44 mm isotropic or less.Acknowledgements
Funding provided by an NSF-GRFP, NIH: R01 NS095985, R01 MH111444, R21 MH116484, S10 RR026351, P41 EB015891, GE Healthcare and the Dana Foundation.References
1. Wang Y, Zhou Y, Wang H, Cui J, Nguchu BA, Zhang X, Qiu B, Wang X, Zhu M. Voxel-based automated detection of focal cortical dysplasia lesions using diffusion tensor imaging and T2-weighted MRI data. Epilepsy Behav 2018;84:127-134.
2. Teipel SJ, Grothe MJ, Filippi M, Fellgiebel A, Dyrba M, Frisoni GB, Meindl T, Bokde AL, Hampel H, Kloppel S and others. Fractional anisotropy changes in Alzheimer's disease depend on the underlying fiber tract architecture: a multiparametric DTI study using joint independent component analysis. J Alzheimers Dis 2014;41(1):69-83.
3. Yang G, Tian Q, Leuze C, Wintermark M, McNab JA. Double diffusion encoding MRI for the clinic. Magn Reson Med 2018;80(2):507-520.
4. van der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. NeuroImage 2008;40(2):559-569.
5. Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016;125:1063-78.
6. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20(2):870-88.
7. Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT and others. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31(3):968-80.
Figures