3479
Diffusion Weighted Imaging with Apparent Diffusion Coefficient on the Assessment of Tumor Heterogeneity for the Differentiation of Molecular Subtypes in Breast CancerJoao Vicente Horvat1, Olivia Sutton1, Blanca Bernard-Davila1, Elizabeth Morris1, Katja Pinker1, and Sunitha Thakur1
1Memorial Sloan Kettering Cancer Center, new york, NY, United States
Synopsis
The
study evaluated the utility of diffusion weighted imaging with apparent
diffusion coefficient on the assessment of tumor heterogeneity for the differentiation
of molecular subtypes in a population of 91 patients with invasive breast
cancer. The authors investigated the use of histogram analysis and visual
assessment of tumor heterogeneity for the differentiation of breast cancer
subtypes derived via immunohistochemistry surrogates. The results obtained
demonstrated that HER-2 enriched tumors had higher ADC values than other tumor
subtypes. It was also demonstrated that histogram analysis and visual
assessment of tumor heterogeneity could not reliably be used to differentiate
tumor molecular subtypes.
Introduction
The heterogeneity in breast cancer is related to aggressiveness and poor prognosis (1). Tumor aggressiveness can also be assessed based on gene-expression profiling, which establishes a classification of breast tumors in four different molecular subtypes: luminal A; luminal B; HER2-enriched; and triple negative/basal-like (2-4). More aggressive tumor subtypes, like Her2-enriched and triple negative, have a propensity for metastatic disease and thus require effective treatment (5-7). Diffusion-weighted imaging (DWI) measures the random motion of water molecules with diffusivity providing a surrogate marker for tissue cellularity, microstructure and tumor heterogeneity (8, 9). The diffusivity can be quantified by calculation of the apparent diffusion coefficient (ADC) and previous studies show that ADC values are associated with tumor aggressiveness and prognosis (10-13). Tumor heterogeneity on DWI can be qualitatively evaluated with visual assessment, yet it is subjective and may be prone to inter- and intra-observer variability. Histogram analysis, a method with which one can quantitatively evaluate the distribution of different ADC values within a lesion, may be used to characterize tumor heterogeneity and thereby identify more aggressive molecular breast cancer subtypes. In this context, the aim of this study was to investigate if quantitative objective histogram analysis or qualitatively visual assessment of tumor heterogeneity on DWI could differentiate molecular subtypes of invasive breast cancers.Methods
In this Health Insurance Portability and Accountability Act compliant and Institutional Review Board approved study, we retrospectively selected consecutive patients from January 2011 to January 2013 with invasive ductal carcinoma of the breast who underwent MRI with DWI with ADC mapping at our institution. The exclusion criteria were 1) lesion with less than 1 cm, 2) previous treatment for breast cancer, 3) pathology report unavailable, and 4) poor DWI image quality. A total of 91 patients were included in the study. Two radiologists specializing in breast imaging independently evaluated the MRI studies. In patients with more than 1 lesion, only the largest was evaluated. A region of interest was drawn on ADC maps in consensus on the slice with the largest diameter covering the whole lesion, taking care to avoid biopsy markers. Histogram analyses was then performed and mean, standard deviation, median, 25% quartile, 75% quartile, 10% quartile, 90% quartile, kurtosis and skewness of ADC values were calculated. For the qualitative analysis, breast tumors were first visualized on contrast-enhanced T1-weighted images and then on DWI and classified according to the degree of heterogeneity. Tumor heterogeneity on DWI was graded on a scale from one to four, being 1=homogeneous, 2=mildly heterogeneous, 3=moderately heterogeneous, and 4=highly heterogeneous. Molecular breast cancer subtypes were derived via IHC surrogates. Tumors were classified as luminal A if either ER or PR was positive and HER2 was negative, Luminal B if either ER or PR was positive and HER2 positive, HER2-enriched if ER and PR were negative and HER2 positive and basal-like if ER, PR and HER2 were negative. Mann-Whitney statistical tests were used to compare quantitative and qualitative results among different molecular subtypes.Results
The ADC values from HER2-enriched tumors were greater than other subtypes, and were statistically significant for ADC 75% and ADC 90% quartiles when compared to luminal tumors (p=0.0180 and 0.0197, respectively) and when compared to triple negative/basal-like (p=0.0429 for both). The histogram analysis quantitative markers of heterogeneity, which included standard deviation, kurtosis and skewness, were not statistically significantly different among molecular tumor subtypes (Figure 1). Qualitative visual assessment of tumor heterogeneity on DWI independently performed by two radiologists demonstrated a greater heterogeneity of more aggressive molecular subtypes, i.e. HER2-enriched and triple negative/basal-like tumors compared to luminal cancers. However, there was also no statistically significant difference among molecular subtypes with respect to heterogeneity classification (Figures 2 and 3).Discussion
Previous studies have investigated histogram analysis of breast lesions for differentiation of benign from malignant lesions according to heterogeneity (14). Whereas there are differences in heterogeneity between benign and malignant lesions, gradations of tumor heterogeneity within molecular subtypes on DWI have not yet been investigated. Our results are in agreement with a previous study by Park et al. (15) which did not show a significant difference on kurtosis and skewness between less aggressive ductal carcinoma in situ and invasive carcinoma. However, with the advent of high-throughput methods to extract multiple imaging parameters, a more sophisticated assessment of tumor heterogeneity through radiomics analysis of other functional MRI parameters might be possible and further studies are warranted.Conclusion
In conclusion, neither quantitative objective histogram analysis nor qualitatively visual assessment of tumor heterogeneity on DWI can differentiate molecular subtypes of invasive breast cancers.Acknowledgements
This study received funding from the NIH/NCI Cancer Center Support Grant (P30CA008748), DOD BCRP W81XWH-09-1-0042 grant, the Breast Cancer Research Foundation and the Susan G. Komen Breast Cancer Foundation.References
1. Suo S, Cheng F, Cao M, Kang J, Wang M, Hua J, et al. Multiparametric diffusion-weighted imaging in breast lesions: Association with pathologic diagnosis and prognostic factors. Journal of magnetic resonance imaging : JMRI. 2017;46(3):740-50. 2. Mazurowski MA, Zhang J, Grimm LJ, Yoon SC, Silber JI. Radiogenomic analysis of breast cancer: luminal B molecular subtype is associated with enhancement dynamics at MR imaging. Radiology. 2014;273(2):365-72. 3. Huber KE, Carey LA, Wazer DE. Breast cancer molecular subtypes in patients with locally advanced disease: impact on prognosis, patterns of recurrence, and response to therapy. Semin Radiat Oncol. 2009;19(4):204-10. 4. Lam SW, Jimenez CR, Boven E. Breast cancer classification by proteomic technologies: current state of knowledge. Cancer treatment reviews. 2014;40(1):129-38. 5. Ko ES, Kim JH, Lim Y, Han BK, Cho EY, Nam SJ. Assessment of Invasive Breast Cancer Heterogeneity Using Whole-Tumor Magnetic Resonance Imaging Texture Analysis: Correlations With Detailed Pathological Findings. Medicine (Baltimore). 2016;95(3):e2453. 6. Fan M, He T, Zhang P, Zhang J, Li L. Heterogeneity of Diffusion-Weighted Imaging in Tumours and the Surrounding Stroma for Prediction of Ki-67 Proliferation Status in Breast Cancer. Sci Rep. 2017;7(1):2875. 7. Li X, Kang H, Arlinghaus LR, Abramson RG, Chakravarthy AB, Abramson VG, et al. Analyzing Spatial Heterogeneity in DCE- and DW-MRI Parametric Maps to Optimize Prediction of Pathologic Response to Neoadjuvant Chemotherapy in Breast Cancer. Transl Oncol. 2014;7(1):14-22. 8. Hassanzadeh E, Glazer DI, Dunne RM, Fennessy FM, Harisinghani MG, Tempany CM. Prostate imaging reporting and data system version 2 (PI-RADS v2): a pictorial review. Abdominal radiology (New York). 2017;42(1):278-89. 9. Partridge SC, McDonald ES. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn Reson Imaging Clin N Am. 2013;21(3):601-24. 10. Durando M, Gennaro L, Cho GY, Giri DD, Gnanasigamani MM, Patil S, et al. Quantitative apparent diffusion coefficient measurement obtained by 3.0Tesla MRI as a potential noninvasive marker of tumor aggressiveness in breast cancer. European journal of radiology. 2016;85(9):1651-8. 11. Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. Journal of magnetic resonance imaging : JMRI. 2009;30(3):615-20. 12. Park SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. Journal of magnetic resonance imaging : JMRI. 2015;41(1):175-82. 13. Costantini M, Belli P, Rinaldi P, Bufi E, Giardina G, Franceschini G, et al. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clinical radiology. 2010;65(12):1005-12. 14. Nogueira L, Brandao S, Matos E, Nunes RG, Loureiro J, Ramos I, et al. Application of the diffusion kurtosis model for the study of breast lesions. European radiology. 2014;24(6):1197-203. 15. Park GE, Kim SH, Kim EJ, Kang BJ, Park MS. Histogram analysis of volume-based apparent diffusion coefficient in breast cancer. Acta Radiol. 2017;58(11):1294-302.Figures
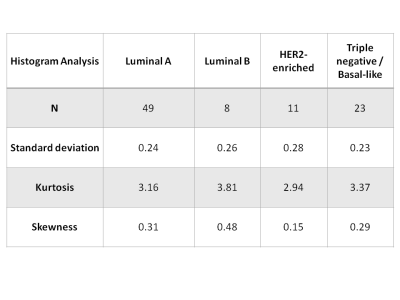
Comparison of histogram analysis markers of
heterogeneity among different molecular subtypes in breast cancer.
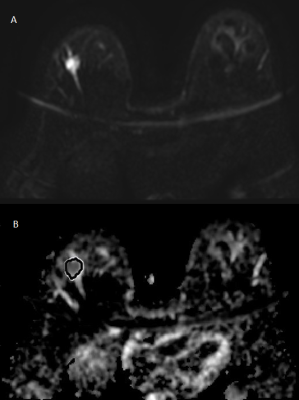
Mildly heterogeneous luminal A tumor on DWI (a)
and ADC map (b) with ROI positioning for histogram analysis.
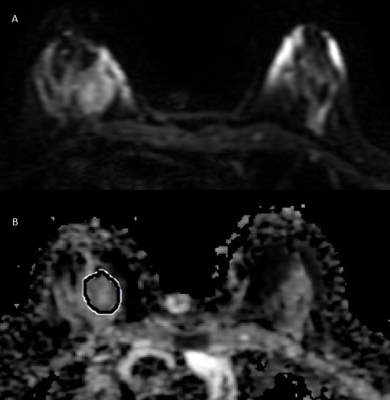
Moderately heterogeneous luminal A tumor on DWI
(a) and ADC map (b) with ROI positioning for histogram analysis.