3369
Is it feasible to directly access the bundle’s specific myelin content, instead of averaging? A study with Microstructure Informed Tractography1Department of Computer Science, University of Verona, Verona, Italy, 2Signal Processing Laboratory 5 (LTS5), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Radiology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, 4FIDMAG Germanes Hospitalàries Research Foundation, Sant Boi de Llobregat, Barcelona, Spain
Synopsis
Diffusion MRI connectometry is a widely used tool to investigate features of structural connectomes that reflect differences in white matter tracks integrity. It consists in averaging microstructural tissues properties (obtained from any voxel-wise map) along streamlines recovered with diffusion tractography. Nevertheless, the average of a microstructural measure is a weak information about an entire bundle. Using microstructure-informed tractography (COMMIT), we were able to simultaneously estimate fiber’s specific myelin water fraction, intra-axonal volume fraction, and g-ratio. We also computed new connectomes with bundles’ specific measures instead of the commonly used averages.
Purpose
Diffusion MRI (dMRI) connectometry1 is a widely used tool to investigate features of structural connectomes that reflect differences in white-matter (WM) tracks integrity between healthy subjects and neurological patients. Its potential is due to the capability of averaging microstructural tissue properties (obtained from voxel-wise maps of any imaging modality) along streamlines recovered with tractography. Nonetheless, the average of a microstructural measure is a weak information about an entire bundle of streamlines if this crosses with other bundles in a significant portion of voxels. The recently proposed COMMIT2-4 framework offers the possibility to recover bundle’s specific tissue properties by estimating the actual contribution to the signal of each individual streamline. The only hypothesis behind this framework is that the microstructural property under investigation is constant along a given streamline, which is the representative trajectory of a coherent set of axons, and additive in each voxel traversed by the streamlines. COMMIT has proven very effective in filtering out false-positive connections and/or estimating dMRI-derived parameters of each bundle (such as intra-axonal signal fractions3, apparent axonal diameters5, etc.). Here, we propose to extend COMMIT by considering simultaneously the intra-axonal volume fraction (AVF), that can be estimated from the dMRI signal using e.g. SMT6 or NODDI7, and the myelin water fraction (MWF), that can be estimated e.g. from quantitative magnetization transfer (qMT)8 or multi-echo T2 images9,10. This allows us, for the first time, to decouple the intrinsic AVF and MWF contributions of each individual streamline in the input tractogram.Methods
Proposed approach. The Convex Optimization Modeling for Microstructure Informed Tractography (COMMIT)2-4 is a framework to complement tractography with additional microstructural information about the neuronal tissue. Its originality lies in the possibility to express tractography and tissue microstructure in a unified formulation using convex optimization. The observation model is $$$\mathbf{y}=\mathbf{Ax}+\eta$$$, where the matrix $$$\mathbf{A}$$$ implements the specific multi-compartments model adopted to characterize the neuronal tissue, $$$\mathbf{x}$$$ are the contributions that are needed to explain the input data $$$\mathbf{y}$$$ and $$$\eta$$$ represents noise. In this work, we implemented a simple forward-model that assigns a signal contribution to every streamline proportionally to its length inside each voxel. Our input data $$$\mathbf{y}$$$ includes both AVF and MWF maps, so the total amount of streamlines traversing a voxel must sum up to, simultaneously, the AVF and MWF values estimated in it. To estimate the individual AVF and MWF contributions of each streamline, while coupling their values, non-negative least-squares with group lasso regularization can be used as in 4: $$$\text{argmin}_{\mathbf{x}\geq 0}||\mathbf{Ax}-\mathbf{y}||_2^2+λ\sum_{g\in G}||\mathbf{x}^g||_2$$$.
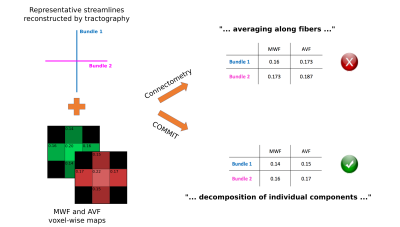
Experimental settings. For illustrative purposes, we tested our proposed approach on a synthetic phantom with two crossing bundles having different MWF and AVF intrinsic contributions (Figure 1). We also tested it on in-vivo data acquired on a healthy volunteer with a Philips 3T scanner. We computed the connectivity matrices from the estimated MWF and AVF contributions of each bundle and compared them with the corresponding averaged values obtained with connectometry.
Results and Discussion
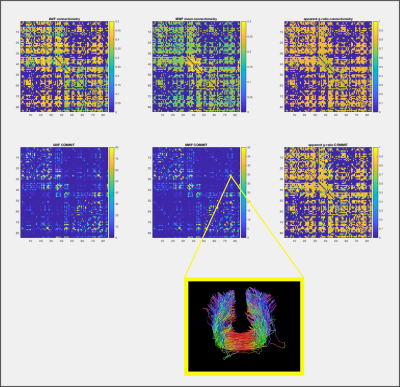
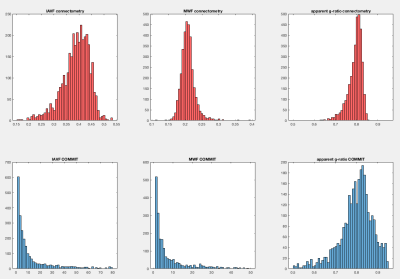
Figure 2 reports the results obtained by using connectometry (average of MWF and AVF along the fibers) and our proposed method (decomposition of MWF and AVF on each streamline by assigning their own specific values). Results clearly show that COMMIT is able to estimate the correct AVF and MWF of each individual streamline, while the values computed with connectometry are biased and different from the ground truth. Figure 3 shows the connectomes resulting from our preliminary test with in-vivo data; the corresponding histograms are reported in Figure 4. As can be seen, the connectometry matrices (top row) are rather flat, a clear consequence of the averaging operation, whereas in the COMMIT matrices we can distinguish bundles with more AVF and/or more MWF. In both cases the g-ratio is overestimated (about 0.8) and flat, but in COMMIT we see smaller values for some bundles. This overestimation could be due to a non-optimal derivation of voxel-wise AVF and MWF; more profound investigations should be conducted on this aspect. Nevertheless, the values estimated with our approach are in line with values found in 11: intra-hemispheric connections seem the more myelinated, while for the inter-hemispheric we found a high value for the anterior frontal bundle.Conclusions
By using the Microstructure Informed Tractography formalism we were able, for the first time, to estimate simultaneously fiber’s specific MWF and AVF for individual bundles in the brain. We speculate that, thanks to the flexibility of COMMIT, it is possible to estimate also the specific g-ratio of axons along each streamline. These novel measures, if confirmed with future experiments on new data, might open the door for a unique and very sensitive tool to study axonal degeneration and/or demyelination in neurological diseases such as multiple sclerosis.Acknowledgements
This work was supported by the Rita Levi Montalcini Programme of the Italian Ministry of Education, University and Research (MIUR), as well as the Instituto de Salud Carlos III (Research project grant: PI15/00277 to ECR). Marco Pizzolato is supported by the Swiss National Science Foundation under grant number CRSII5_170873 (Sinergia project).
References
1 - F.C. Yeh, P.F. Tang, W.Y. Isaac Tseng. Diffusion MRI connectometry automatically reveals affected fiber pathways in individuals with chronic stroke. NeuroImage Clin., 2:912-921, 2013
2 - A. Daducci, A. Dal Palù, A. Lemkaddem and J.P. Thiran. A convex optimization framework for global tractography. In Proc. IEEE ISBI, 524–7, 2013
3 - A. Daducci, A. Dal Palù, A. Lemkaddem, J.P. Thiran. COMMIT: Convex Optimization Modeling for Microstructure Informed Tractography, IEEE Trans. Med. Imaging., 33:246-57, 2014
4 - A. Daducci, M. Barakovic, G. Girard, M. Descoteaux, and J.P. Thiran. Reducing false positives in tractography with microstructural and anatomical priors. Proc. Int. Soc. Magn. Reson. Med. 26, 0038, 2018
5 - M. Barakovic, G. Girard, D. Romascano, J. Rafael-Patino, M. Descoteaux, G. Innocenti, D.K. Jones, J.P. Thiran, A. Daducci. Assessing feasibility and reproducibility of a bundle-specific framework on in vivo axon diameter estimates at 300mT/m. Proc. Int. Soc. Magn. Reson. Med. 26, 5245, 2018
6 - E. Kaden, N.D. Kelm, R.P. Carson, M.D. Does, D.C. Alexander. Multi-compartment microscopic diffusion imaging. NeuroImage, 139:346-359, 2016
7 - G.H. Zhang, T. Schneider, C.A.Wheeler-Kingshott, D.C. Alexander. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage, 61:1000-1016, 2012
8 - J.G. Sled, B.G. Pike. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magn. Reson. Med., 46:923-93, 2001
9 - E. Canales-Rodriguez, M. Pizzolato, Y. Alemán-Gómez, N. Kunz, C. Pot, J.P. Thiran, A. Daducci. Unified multi-modal characterization of microstructural parameters of brain tissue using diffusion MRI and multi-echo T2 data. Proc. Int. Soc. Magn. Reson. Med. 26, 5234, 2018
10 - M. Pizzolato, E. Canales-Rodriguez, A. Daducci, J.P. Thiran. Multimodal microstructure imaging: joint T2-relaxometry and diffusometry to estimate myelin, intracellular, extracellular and cerebrospinal fluid properties. Proc. Int. Soc. Magn. Reson. Med. 26, 3118, 2018
11 - N. Stikov, L.M. Perry, A. Mezer, E. Rykhlevskaia, B.A. Wandell, J.M. Pauly, R.F. Dougherty. Bound pool fractions complement diffusion measures to describe white matter micro and macrostructure. NeuroImage, 54:1112-1121, 2011
Figures