3329
Magnetic resonance neurography reveals association between low serum cholesterol and peripheral nerve damage in type 2 diabetes1Heidelberg University Hospital, Heidelberg, Germany, 2Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany, 3Neuroradiology, Würzburg University Hospital, Würzburg, Germany
Synopsis
Diabetic polyneuropathy (DPN) is a major contributor for morbidity in diabetes; however, up to this day, there is a lack of sufficient strategies to prevent this disorder especially in patients suffering from type 2 diabetes (T2D). Specifically, it remains controversially discussed if a lowering of serum cholesterol has beneficial effects on the course of T2D DPN. Using in vivo high resolution magnetic resonance neurography in 100 type 2 diabetes patients with and without DPN, we found that lowering of serum cholesterol, especially lowering of LDL, is strongly associated with an increase in visible nerve damage. We further found a significant correlation between the amount of nerve damage and the patients’ statin dose. In T2D patients, this effect is potentially relevant for therapies that promote an aggressive lowering of serum cholesterol.
Introduction
Distal symmetric diabetic polyneuropathy (DPN) is an increasingly prevalent severe and frequent complication of diabetes mellitus; however, DPN pathophysiology is not completely understood and therapeutic management often has a poor outcome, specifically for patients with type 2 diabetes (T2D). Prior works could identify risk factors for the development of DPN in T2D that are associated with dyslipidemia, such as low levels of high density lipoprotein (HDL) [1]. Yet it remains unclear if a lowering of low-density lipoprotein (LDL) and total serum cholesterol, or prolonged statin therapy has beneficial effects on patients that suffer from T2D [2]. This study aimed to investigate the influence of cholesterol metabolism on structural neural remodeling in T2D DPN in vivo.Material and Methods
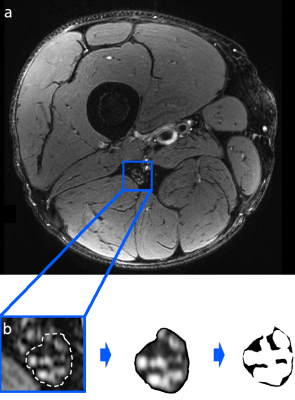
Magnetic resonance neurography of the right thigh was prospectively performed at 3 Tesla in 100 patients with T2D, that had either DPN (n=64) or no DPN (n=36), with a T2-weighted turbo spin echo 2D sequence with spectral fat saturation (T2wFS; TR: 5970 ms, TE: 55 ms, field of view: 160 x 160 mm2, matrix size: 512 x 512, slice thickness: 4 mm, interslice gap: 0.35 mm, voxel size: 0.5 x 0.3 x 4.0 mm3, number of slices: 24). Each patient had an additional, extensive workup where clinical, electrophysiological and serological data were obtained. Nerve segmentation was performed semi-automatically with custom-written code in Matlab v7.14.0.0739(R2012a), and three-dimensional nerve reconstruction was obtained for the sciatic nerve's tibial compartment, see also Fig. 1. The neural lesion load was obtained for T2wFS hypointense, lipid-equivalent intraneural lesions, and subsequently correlated with all acquired parameters.Results
Patients with T2D DPN showed a higher lesion load of T2wFS hypointense lesions (LEL) than patients with no DPN (19.67%±2.03 vs. 10.03±0.87; p<0.001). Furthermore, LEL showed a positive correlation with the nerve's mean cross-sectional area (r=0.44; p<0.001), and a negative association with nerve conduction velocities and amplitudes of the tibial (r=-0.33; p=0.014 and r=-0.31; p=0.020, respectively) and peroneal nerve (r=-0.51; p<0.001 and r=-0.28; p=0.034, respectively). In addition LEL was negatively correlated with total serum cholesterol (r=0.41; p<0.001), HDL cholesterol (r=-0.30; p=0.006), and LDL cholesterol (r=-0.33; p=0.003). In addition, statin dose and LEL were positively correlated (r=0.39; p=0.005), please see also Fig. 2.Discussion and Conclusion
The findings of this study indicate that a lowering of serum cholesterol in T2D DPNis associated with an increase in neural lesions and with a decrease in nerve conduction velocities. This effect may potentially be explained by impairment of peripheral nerve regeneration through lowering of serum cholesterol, since the latter cannot be produced in axons. Instead, cholesterol in regenerating axons needs to be supplied to neurite tips and adjacent Schwann cells either by axonal transport or externally via HDL and LDL [3]. This is specifically relevant with regard to emerging dyslipidemia management strategies in T2D, including novel PCSK9 inhibitors that promote a more pronounced lowering of serum cholesterol [4]. Clinical trials that study the effects of low serum cholesterol in T2D should therefore pay close attention to potential signs of neuropathy.Acknowledgements
The study received funding from the German Research Foundation (Grant numbers SFB 1158 and SFB 1118).References
[1] Jende JME, Groener JB, et al. Diabetic neuropathy differs between type 1 and type 2 diabetes: Insights from magnetic resonance neurography. Ann Neurol, 83:588-598, 2018.
[2] Gaist D, Jeppesen U, et al. Statins and risk of polyneuropathy: a case-control study. Neurology, 58:1333-1337, 2002.
[3] de Chaves EI, Rusiñol AE, et al. Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J Biol Chem, 272:30766-30773, 1997.
[4] Segoviano-Mendoza M, Cardenas-de la Cruz M, et al. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. J Lipid Res, Springer International Publishing, 4:427-443, 2017.
Figures