3308
Phase imaging and serum neurofilaments: a combined laboratory-imaging marker of multiple sclerosis chronic inflammation1Department of Neurology, Lausanne University Hospital, Lausanne, Switzerland, 2Translational Neuroradiology Section/NINDS, National Institutes of Health, Bethesda, MD, United States, 3Laboratory of Neuroimmunology, Lausanne University Hospital, Lausanne, Switzerland, 4Department of Radiology, Lausanne University Hospital, Lausanne, Switzerland, 5Department of Neurology, Basel University Hospital, Basel, Switzerland, 6Translational Imaging in Neurology (ThINk) Basel, Department of Biomedical Engeneering, Basel University Hospital, Basel, Switzerland
Synopsis
In multiple sclerosis (MS), persistent chronic inflammation at the edges of old non-gadolinium-enhancing white matter lesions, is identified with a paramagnetic rim on susceptibility-based-MRI sequences. Serum neurofilaments (sNfL) levels are associated with disease activity and neurodegeneration in acute and chronic phases of MS. Whether the presence of chronic inflammation is accompanied by increased in neuroaxonal destruction is currently unknown. We showed that MS patients featuring chronic inflammation at the lesions edges have higher neuroaxonal destruction than patients without. The combination of paramagnetic rim and sNfL may help in the selection of “chronically” active MS patients who may benefit from disease-modifying-treatments.
Introduction
In multiple sclerosis (MS), persistent chronic inflammation and remyelination failure at the edges of old non-gadolinium (Gd)-enhancing white matter (WM) lesions, is identified with a paramagnetic rim on susceptibility-based MRI sequences (1, 2). On the other hand, serum neurofilaments (sNfL) levels are associated with disease activity and neurodegeneration in both acute and chronic phases of MS (3, 4). Yet, whether the presence of chronic (“smoldering”) inflammation (as measured by susceptibility-based MRI) is accompanied by increased in neuroaxonal destruction (as assessed by sNfL) in living patients is currently unknown. The goal of this study was to assess the relationship between the presence and number of paramagnetic rims and the level of sNfL (3) in MS patients.Methods
Patients with relapsing remitting MS (RRMS) and primary or secondary progressive MS (PMS) (5) were recruited from the Neuroimmunology/MS outpatient center at the University Hospital of Lausanne, Switzerland. Patients featuring visible contrast enhancing lesions on post-Gd T1-weighted images were excluded from the analysis. For each patient, expanded disability status scale (EDSS), blood sample, and MRI data were collected within a time window of 13, 4-22 days (median, IQR). A 3D segmented EPI(6) sequence giving high resolution (0.65 mm3 voxels) T2*-weighted and phase images was acquired during the intravenous injection of Gd on a 3T Siemens (Skyra or Prisma) MRI scanner. Phase postprocessing and image coregistration were performed as previously described (1, 7, 8). Phase images were analyzed independently by two raters (P.M. and M.A.) for the presence of paramagnetic rims in chronic non-Gd enhancing WM lesions, as previously described (8). Serum neurofilament analysis was performed with the Single Molecule Array (Simoa) assay (SIMOA) at the Basel University Hospital, Switzerland (3).Results
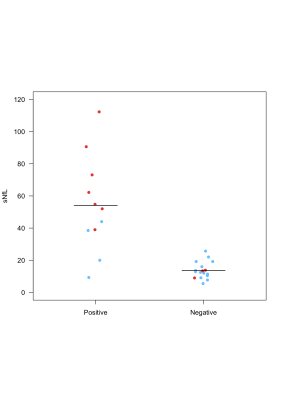
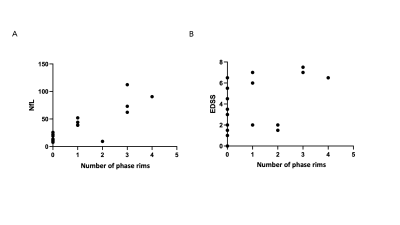
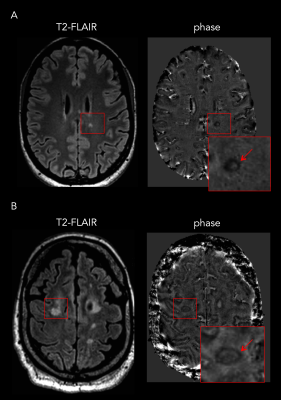
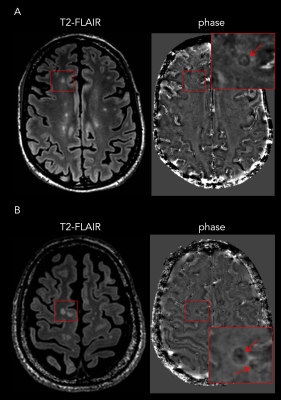
Twenty-eight patients were included (RRMS: n=18 and PMS: n=10). Eleven of 28 patients (39%; “rim-positive”) had at least one chronic WM lesion featuring a paramagnetic rim. The agreement between raters was 91%. The level of sNfL was significantly higher in rim-positive compared to rim-negative cases (mean±SD: 54±29 and 14±5 pg/ml respectively, p<0,0001; Figure 1A). Interestingly, in the subgroup of 10 progressive PMS, the level of sNfL was higher in all rim-positive (n=7) compared to rim-negative (n=3) patients (mean 69±25 and 12±3 pg/ml respectively; Figure 1B). No age difference was observed between rim-positive and rim-negative patients (mean 46±11 and 48±13 years old, respectively). Also, we observed a positive correlation between the number of paramagnetic rims and the level of sNfL (p<0,0001) and between the number of rims and the EDSS per patient (p<0,001; Figure 2). Examples of paramagnetic rims in RRMS, SPMS, and PPMS patients are shown in Figures 3 and 4.Conclusions
Our results show that MS patients featuring chronic smoldering inflammation at the edges of some lesions have higher neuroaxonal destruction than patients without. This is particularly relevant since our cohort of patients did not include subjects with active inflammation as measured by the presence of Gd enhancing lesions. While these results should be confirmed in larger MS cohorts, they suggest that the combination of paramagnetic rim and serum NfL assessment may help in the selection of “chronically” active MS patients who may benefit from disease-modifying treatments.Acknowledgements
No acknowledgement found.References
1. Absinta M, Sati P, Schindler M, Leibovitch EC, Ohayon J, Wu T, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. The Journal of clinical investigation. 2016;126(7):2597-609. 2. Dal-Bianco A, Grabner G, Kronnerwetter C, Weber M, Hoftberger R, Berger T, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathologica. 2017;133(1):25-42. 3. Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Annals of neurology. 2017;81(6):857-70. 4. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nature reviews Neurology. 2018;14(10):577-89. 5. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet neurology. 2018;17(2):162-73. 6. Sati P, Thomasson D, Li N, Pham D, Biassou N, Reich D, et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult Scler. 2014. 7. Absinta M, Sati P, Gaitan MI, Maggi P, Cortese IC, Filippi M, et al. Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Annals of neurology. 2013;74(5):669-78. 8. Absinta M, Sati P, Fechner A, Schindler MK, Nair G, Reich DS. Identification of Chronic Active Multiple Sclerosis Lesions on 3T MRI. AJNR American journal of neuroradiology. 2018.Figures