3209
Reduced cerebral blood flow measured in the EAE mouse model of multiple sclerosis using perfusion MRI1Department of Radiology, University of Calgary, Calgary, AB, Canada, 2Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 3Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada, 4Experimental Imaging Center, University of Calgary, Calgary, AB, Canada
Synopsis
Multiple sclerosis (MS) is a chronic neurological disease involving inflammation. Brain hypoxia, or low brain oxygenation, in MS is an emerging field of study. It has been shown that some MS patients have reduced cerebral blood flow (CBF), but the mechanism is unclear. Experimental autoimmune encephalomyelitis (EAE) is a mouse model used to study inflammation-associated neurodegeneration. Using perfusion MRI and immunohistochemistry, we demonstrated that CBF reduction in EAE may be due to cerebral blood vessel occlusion in response to systemic inflammation. CBF reduction, coupled with brain inflammation, is a likely cause of hypoxia in MS.
Introduction
Multiple sclerosis (MS) is a chronic neurological disease that is characterized by periods of inflammation and demyelination of the central nervous system (CNS). While MS is typically considered a white matter disease, grey matter (GM) pathology is also involved.1 Emerging evidence from both animal model and human studies suggests that GM hypoxia, or low oxygen, is characteristic of MS.2,3,4,5 It is important that we understand the cause of hypoxia, as hypoxia may exacerbate MS inflammation.6 Several MRI and PET experiments have demonstrated that hypoxic stress in MS may result from reduced GM cerebral blood flow (CBF).7,8 Although the mechanism of CBF reduction in MS is not well characterized, one hypothesis is that small cerebral blood vessels become physically occluded in response to inflammation.6,9 We used experimental autoimmune encephalomyelitis (EAE), an animal model of MS, to demonstrate that CBF reduction, and consequent hypoxia, occur in response to inflammation as a result of vascular obstruction by immune cells.Methods
EAE was induced in female C57BL/6 mice using MOG35-55 peptide and mycobacterium emulsified in complete Freund’s adjuvant (CFA) as described previously10; pertussis toxin was administered on days 0 and 2. CFA control mice were subjected to the same treatment except MOG was omitted. A 9.4T MRI with a 35 mm volume coil was used to perform perfusion imaging to measure CBF in mice 14-16 days post-induction (n = 4 naïve, 5 CFA, 7 EAE). To account for magnetization transfer, a four image continuous arterial spin labeling (CASL) sequence was used: TR = 3000ms, TE = 2.66ms, averages: 16, RARE factor = 36, FOV = 30x30 mm, matrix = 128x128, voxel size: 0.23x0.23x1 mm.11 T1 map was obtained using a RARE-VTR sequence: TR=100, 500, 1000, 3000, 7500 ms, TE=10 ms. To compare CBF between groups, a t-test with a Bonferroni correction was used.
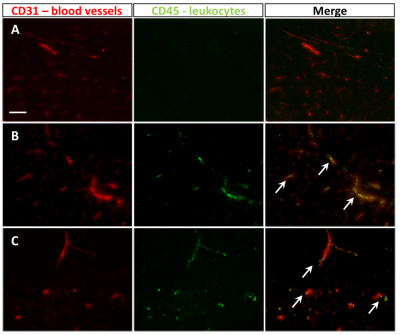
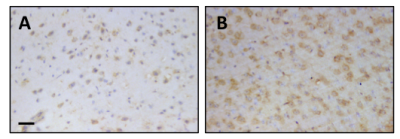
Following imaging, on day 16 post-induction, mice were sacrificed and brains were extracted for histology. Immunohistochemistry was done to look for occlusion of blood vessels. Blood vessels were stained with an anti-CD31 antibody while leukocytes were detected by CD45. A DAB-enhanced pimonidazole procedure was used to detect hypoxia.12 Images were taken using a light microscope.
Results
CBF for naïve mice was 200±22 ml/100g/min, for CFA mice was 153±34 ml/100g/min, and for EAE mice was 164±22 ml/100g/min (mean±SD; Figure 1). CBF was significantly reduced in both CFA (p=0.048) and EAE (p=0.028) mice compared to naïve. There was no significant difference between CFA and EAE mice. CD45/CD31 immunofluorescence staining showed leukocyte aggregation in CFA and EAE, but not naïve, cortical blood vessels (Figure 2). Positive staining for pimonidazole was observed in the cortex of CFA and EAE mice but was more evident in EAE mice (Figure 3).Discussion
We observed a reduction in GM CBF, similar to that seen in MS, in EAE using perfusion MRI. This suggests that inflammation may be causing a change in blood flow. Unexpectedly, we found reduced CBF in CFA controls. As mycobacterium and pertussis toxin act as immune system stimulants, increased systemic inflammation may cause physical obstruction of cerebral blood vessels in CFA and EAE mice. This hypothesis is supported by our CD45/CD31 staining, which demonstrates leukocyte aggregation in CFA and EAE vessels (Figure 2). Similar observations have been made in ischemic stroke literature. Burrows et al. demonstrated that administration of pro-inflammatory cytokines following occlusion removal results in reduced tissue reperfusion, due to immune cell accumulation in microvessels.13 It is possible that a similar phenomenon is contributing to MS pathology.
The fact that EAE mice are more hypoxic compared to CFA controls could be attributed to the different inflammatory processes that were stimulated. CFA does not trigger an autoimmune response; thus, inflammation is a generalized systemic response not involving brain parenchyma. Conversely, we expect both systemic and parenchymal inflammation in EAE mice. CNS inflammation in EAE induces demyelination, which causes energy and oxygen demand to surpass homeostatic supply.14 Therefore, more extensive hypoxia in EAE mice may be a result of the combined effects of reduced CBF and CNS inflammation.
Conclusion
As far as we are aware, we are the first group to use perfusion MRI to demonstrate reduced GM CBF in CFA mice. Reduced CBF was observed in both immune-stimulated CFA and EAE animals, and immunohistological staining indicated leukocyte aggregation. Therefore, we hypothesize that physical occlusion of blood vessels contributes to brain hypoxia, and this hypoxia may become more extensive with parenchymal inflammation, as in EAE and MS. This re-opens the door for understanding how oxygen-related therapies may reduce injury and promote recovery in inflammation-associated neurodegeneration.Acknowledgements
Funding for this study was provided by the AIHS CRIO team grant.References
1. Haider L, Simeonidou C, Steinberger G, et al. Multiple sclerosis deep grey matter: the relationship between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014;85(12):1386-95.
2. Johnson TW, Wu Y, Nathoo N, et al. Gray matter hypoxia in the brain of the experimental autoimmune encephalomyelitis model of multiple sclerosis. PLOS ONE. 2016;11(12):e0167196.
3. Davies AL, Desai RA, Bloomfield PS, et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann Neurol. 2013;74(6):815-25.
4. Yang R & Dunn JF. Reduced cortical microvascular oxygenation in multiple sclerosis: A blinded, case-controlled study using a novel quantitative near-infrared spectroscopy method. Sci Rep. 2015;5:16477.
5. Nathoo N, Agrawal S, Wu Y, et al. Susceptibility-weighted imaging in the experimental autoimmune encephalomyelitis model of multiple sclerosis indicates elevated deoxyhemoglobin, iron deposition and demyelination. Mult Scler. 2012;19(6):721-31.
6. Yang R & Dunn JF. Multiple sclerosis disease progression: Contributions from a hypoxia-inflammation cycle. Mult Scler. 2018;1352458518791683.
7. Sun X, Tanaka M, Kondo S, et al. Clinical significance of reduced cerebral metabolism in multiple sclerosis: A combined PET and MRI study. Ann Nucl Med. 1998;12(2):89-94.
8. Varga AW, Johnson G, Babb JS, et al. White matter hemodynamic abnormalities precede sub-cortical gray matter changes in multiple sclerosis. J Neurol Sci. 2009;282(1-2):28-33.
9. Williams R, Rohr AM, Wang W, et al. Iron deposition is independent of cellular inflammation in a cerebral model of multiple sclerosis. BMC Neurosci.2011;12:59.
10. Agrawal SM, Silva C, Tourtellotte WW & Yong VW. EMMPRIN: A novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci. 2011; 31: 669-77.
11. Lei H, Grinberg O, Nwaigwe CI, et al. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913(2):174-9.
12. Arteel GE, Thurman RG & Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state.Eur J Biochem. 1998;253:743-50.
13. Burrows F, Haley MJ, Scott E et al. Systemic inflammation affects reperfusion following transient cerebral ischemia. Exp Neurol. 2016;277:252-60.
14. Desai RA & Smith KJ. Experimental autoimmune encephalomyelitis from a tissue energy perspective. F1000Res. 2017;6:1973.Figures