3186
Amyloid- β Associations with White Matter Integrity in Down Syndrome Assessed Using Diffusion Tensor Imaging and 11C-PiB Positron Emission Tomography1University of Wisconsin-Madison, Madison, WI, United States, 2University of Pittsburgh, Pittsburgh, PA, United States, 3Columbia University, New York, NY, United States, 4University of Cambridge, Cambridge, United Kingdom
Synopsis
Nearly all people with Down Syndrome will develop Alzheimer’s Dementia neuropathology by age 50, often asymptomatically. In this work, diffusion tensor imaging (DTI) was used to characterize white matter (WM) tract microstructure in thirty-three non-demented participants with Down Syndrome. Amyloid plaque burden was assessed using PET imaging with C-11 PiB. DTI measures were used to compare WM microstructure in DS individuals with high amyloid burden (PiB+) versus individuals with low amyloid burden (PIB-). PIB+ adults demonstrated significantly increased mean diffusivity and decreased fractional anisotropy in several bilateral WM regions. These findings are consistent of signs of WM degeneration.
Introduction
Nearly all persons with Down Syndrome will show disease pathology of Alzheimer’s dementia by age 50.1 It has been observed that the accumulation of amyloid-β (Aβ) proteins in the form of plaques is linked to the neuropathology and onset of Alzheimer’s disease (AD).2 Further, the positron emission tomography (PET) radiotracer, Pittsburgh Compound B (PiB) has been observed to be retained in areas of increased Aβ plaques, making PiB an effective biomarker of Alzheimer’s neuropathology.3 Diffusion tensor imaging (DTI) characterizes the microstructure of brain tissue, especially white matter, by quantifying the amount and direction of diffusion of water within the brain. In this work, we set out to investigate the effects of Aβ on white matter integrity, as well as gray matter, in a cohort of 33 individuals with Down Syndrome who were assigned a score of PiB+ or PiB- based on theaccumulation of C-11 PiB. We compare DTI scalar maps, namely fractional anisotropy (FA) and mean diffusivity (MD) in the PiB+ and PiB- cohorts.Methods
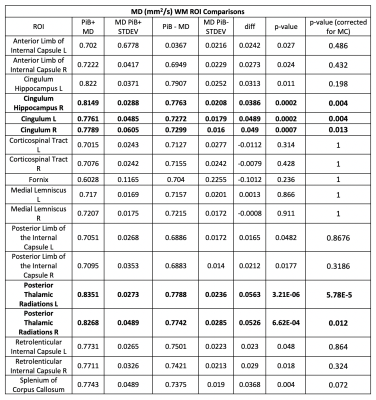
A cohort of forty (n=40) adults with Down Syndrome were scanned with both PET and MRI. T1- weighted structural MRI, and DTI scans were acquired at 3T. The DTI protocol consisted of a single shell, b=1000s/mm2, with 52 encoding directions and 1.875mmx 1.875mm x 2.500mm resolution.Data were processed using an in-house processing pipeline utilizing DIPY, FSL4, and MRtrix5. Processing included corrections for Rician noise6, Gibbs ringing7, eddy-currents8,9, and susceptibility distortions. The tensor model was then fitted using the DiPy toolkit. Due to errors with field-map acquisitions, a total of 7 participants were excluded, leaving a total of thirty- three (n=33) usable subjects. FA maps for each subject were spatially normalized and up- sampled to the FMRIB b58 1mm FA template10; subject-specific warps were then applied to the MD maps.PET scans were acquired on a Siemens ECAT EXACT HR+PET scanner. Up to 15mCi of C-11 PiB were delivered via slow bolus injection. Standard uptake value ratios (SUVR) were used to label participants as PiB+ or PiB- in a process previously described.11,12 Of the 33 participants, 8 were labelled PiB+ and 25 were labelled PiB-13.After alignment, comparisons of PIB+ versus PIB-, FA and MD values within 18 regions of interest (ROIs) extracted from the JHU WM structural atlas14 were made. Averages were calculated within each ROI and statistical testing with Bonferroni multiple comparisons correction (factor of 18) was used.Results
In Table 1, we observed significant bilateral decreases in FA of the corpus callosum, retrolenticular and posterior limbs of the internal capsule, posterior thalamic radiations, the cingulum, and the hippocampal portion of the cingulum. In Table 2, we observe increased MD in the PIB+ cohort in the same bilateral WM ROIs as the decreased FA, plus the anterior limb of the internal capsule.Discussion
In this study, we were able to successfully acquire DTI data in a cohort of 33 adults with Down Syndrome. Although, neuroimaging studies of Down Syndrome are challenging, careful quality control and minor correction of the raw DWI data provided useable DTI measures. As predicted, the Down Syndrome cohort with higher prevalence of amyloid burden in the brain demonstrated decreased FA and increased MD in multiple white matter regions. The relationship between amyloid burden from C11 PIB and DTI white matter changes are consistent with observations in studies of aging cohorts at risk for Alzheimer’s disease15,16,17. Future studies will examine the relationships between amyloid burden, white matter microstructure, and cognitive impairment.Limitations: While this is one of the larger DTI studies of Down Syndrome to date, the sample size remains fairly small when the cohort is split into PIB-/+ subgroups. Sample size issues also make it impossible to assess the effects of sex or to disambiguate the effects of age and amyloid burden as amyloid burden increases with age.Acknowledgements
No acknowledgement found.References
1,11,13Lao, P. J., Handen, B. L., Betthauser, T. J., Mihaila, I., Hartley, S. L., Cohen, A. D., Tudorascu, D. L., Bulova, P. D., Lopresti, B. J., Tumuluru, R. V., Murali, D., Mathis, C. A., Barnhart, T. E., Stone, C. K., Price, J. C., Devenny, D. A., Mailick, M. R., Klunk, W. E., Johnson, S. C., ... Christian, B. T. (2017). Longitudinal changes in amyloid positron emission tomography and volumetric magnetic resonance imaging in the nondemented Down syndrome population. Alzheimer's & dementia (Amsterdam, Netherlands), 9, 1-9. doi:10.1016/j.dadm.2017.05.001
2Wisniewski K.E., Wisniewski H.M., Wen G.Y. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278–282
3Klunk, W. E., Engler, H., Nordberg, A., Wang, Y., Blomqvist, G., Holt, D. P., ... & Ausén, B. (2004). Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 55(3), 306-319.
4,10M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62:782-90, 2012
5Tournier, J. D., Calamante, F., & Connelly, A. (2012). MRtrix: diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology, 22(1), 53-66.
6Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394-406.
7Kellner, E; Dhital, B; Kiselev, V.G & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine, 2016, 76, 1574–1581.
8Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-78.
9Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556-72
12Cohen A.D., Mowrey W., Weissfel L.A., Aizenstein H.J., McDade E., Mountz J.M. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–215
14Oishi, K., Zilles, K., Amunts, K., Faria, A., Jiang, H., Li, X., Akhter, K., Hua, K., Woods, R., Toga, A. W., Pike, G. B., Rosa-Neto, P., Evans, A., Zhang, J., Huang, H., Miller, M. I., van Zijl, P. C., Mazziotta, J., ... Mori, S. (2008). Human brain white matter atlas: identification and assignment of common anatomical structures in superficial whitematter. NeuroImage, 43(3), 447-57.
15Mayo, C. D., Mazerolle, E. L., Ritchie, L., Fisk, J. D., Gawryluk, J. R., Alzheimer's Disease Neuroimaging Initiative (2016). Longitudinal changes in microstructural white matter metrics in Alzheimer's disease. NeuroImage. Clinical, 13, 330-338. doi:10.1016/j.nicl.2016.12.012
16Teipel, S. J., Grothe, M. J., Filippi, M., Fellgiebel, A., Dyrba, M., Frisoni, G. B., ... & Hauenstein, K. (2014). Fractional anisotropy changes in Alzheimer's disease depend on the underlying fiber tract architecture: a multiparametric DTI study using joint independent component analysis. Journal of Alzheimer's Disease, 41(1), 69-83.
17Stebbins, G. T., & Murphy, C. M. (2009). Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behavioural neurology, 21(1, 2), 39-49.
Figures