3045
Increased water-exchange across the blood-brain barrier in spontaneously hypertensive rats1Division of Neuroscience and Experimental Psychology, University of Manchester, Manchester, United Kingdom, 2Bioxydyn Ltd, Manchester, United Kingdom
Synopsis
Blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) dysfunction are increasingly recognised as pathological hallmarks of vascular dementia and Alzheimer’s disease. Chronic hypertension increases the risk of developing both types of dementia, and may contribute by disrupting the function of blood-brain interfaces. Here we study the permeability of blood-brain and blood-CSF barriers to water using our recently developed multi-flip angle multi-echo (MFAME)-MRI protocol in spontaneously hypertensive rats (SHR). SHRs display increased BBB permeability surface area product to water, relative to age matched controls. Such changes may alter brain water and ion balance and/or contribute to glymphatic dysfunction. Blood-CSF barriers were unaffected.
Introduction
Mid-life hypertension increases the risk of developing dementia by approximately 25-30%1. Drainage of interstitial fluid into the ventricular system is altered in hypertensive rats2, which may contribute towards amyloid-β accumulation and cerebral amyloid angiopathy3, possibly through dysfunctional fluid movement across the blood-brain barrier (BBB), blood-cerebrospinal fluid barrier (BCSFB), or through the glymphatic system. At a microscopic level, chronic hypertension leads to altered expression and depolarisation of key BBB, BCSFB, and glial cell transmembrane proteins that nominally regulate the permeability of blood-brain and blood-CSF interfaces, including tight junctions4 and aquaporin water channels5,6. In this study, we use our recently developed multi-flip angle multi-echo (MFAME)-MRI method7 to probe trans-BBB and trans-BCSFB water-exchange in spontaneously hypertensive rats (SHR) and Wistar-Kyoto controls aged 13 months.Methods
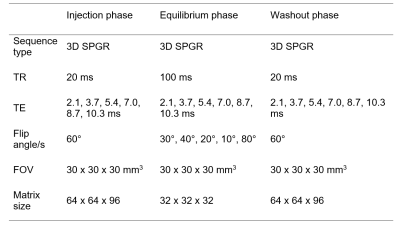
Male spontaneously hypertensive (SHR) rats (n = 7) and age-matched normotensive Wistar-Kyoto (WKY) controls (n = 9) aged 13 months were scanned using a Bruker Avance III console interfaced with an Agilent 7T 16 cm bore magnet. High-resolution T2-RARE images were acquired to enable accurate delineation of brain regions of interest (ROIs) via registration with the Schwarz et al. rat brain atlas8. Ventricle ROIs were generated by thresholding pre-contrast variable flip angle T1 maps (CSF in ventricles was defined as voxels with T1 > 3 s). Measurements of the BBB and BCSFB permeability surface-area product to water (PSw [mL min-1 mL-1]) were made using MFAME-MRI as previously described7. In brief, 3D SPGR images at multiple flip angles were collected following injection of Gd-DOTA (Table 1). Estimates of blood T1 were then extracted from the superior sagittal sinus pre- and post injection and used to estimate PSw from the signal time-course in key ROIs using the two-site one exchange model. Estimates of PSw were log-transformed to improve normality prior to statistical analysis. ANOVA tests were performed on regional measures of BBB ln(PSw) to estimate the effects of group (SHR/WKY) and brain region (repeated measure). Post-host t-tests were performed to determine significance of individual regions. BCSFB estimates of ln(PSw) were tested for SHR/WKY differences using a t-test.Results
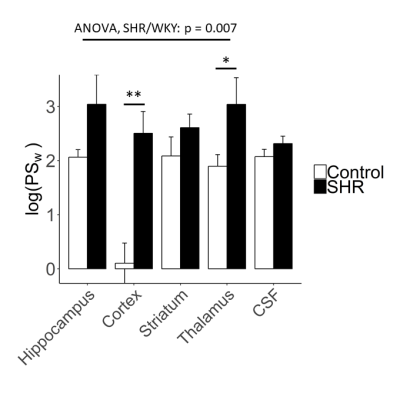
ANOVA analyses showed that BBB PSw was higher in SHR rats than WKY controls (Figure 1, p = 0.0070) and differed significantly across brain regions (p < 10-4). BCSFB PSw did not differ between SHR and WKY rats (p = 0.23). PSw at the BCSFB was similar to PSw at the BBB.Discussion
This is the first report of BBB and BCSFB PSw measurements in SHRs. Our results indicate that water-exchange across the BBB is greater in SHR than normotensive controls, whereas water-exchange across the BCSFB did not differ between strains (Figure 1). Several studies have detected increased aquaporin-4 expression on astrocyte end-feet in hypertensive rats which may contribute to the increased trans-BBB water-exchange observed in this study5,6. Importantly, aquaporin-4 channels are thought to play a key role in maintaining glymphatic flow and aid clearance of waste from the brain9, indicating MFAME-MRI may be indirectly sensitive to glymphatic flow patterns. Increased PSw may also result from subtle reductions in the expression of BBB tight junction proteins, as we have observed in our previous work in transgenic Alzheimer’s disease rats7. In summary we have shown increased BBB PSw in hypertensive rats, which may reflect increased aquaporin-4 expression or subtle tight junction loss at the BBB.Acknowledgements
The purchase, maintenance, and scanning of the SHR rats was supported by Wellcome Trust ISSF scheme and EPSRC project EP/M005909/1. The MRI facility is supported through an equipment grant from BBSRC UK (BB/F011350).References
1. Whitmer, R. A., Sidney, S., Selby, J., et al. (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 64(2), 277-281.
2. Bedussi, B., Naessens, D. M., de Vos, J., et al. (2017). Enhanced interstitial fluid drainage in the hippocampus of spontaneously hypertensive rats. Scientific reports, 7(1), 744.
3. Bueche, C. Z., Hawkes, C., Garz, C., et al. (2014). Hypertension drives parenchymal β‐amyloid accumulation in the brain parenchyma. Annals of clinical and translational neurology, 1(2), 124-129.
4. Fan, Y., Yang, X., Tao, Y., et al. (2015). Tight junction disruption of blood–brain barrier in white matter lesions in chronic hypertensive rats. NeuroReport, 26(17), 1039-1043.
5. Ishida, H., Takemori, K., Dote, K., et al. (2006). Expression of glucose transporter-1 and aquaporin-4 in the cerebral cortex of stroke-prone spontaneously hypertensive rats in relation to the blood-brain barrier function. American journal of hypertension, 19(1), 33-39.
6. Tomassoni, D., Bramanti, V., & Amenta, F. (2010). Expression of aquaporins 1 and 4 in the brain of spontaneously hypertensive rats. Brain research, 1325, 155-163.
7. Dickie, B. R., Vandesquille, M., Ulloa, J., et al. (2019). Water-exchange MRI detects subtle blood-brain barrier breakdown in Alzheimer's disease rats. NeuroImage, 184, 349-358.
8. Schwarz, A. J., Danckaert, A., Reese, T., et al. (2006). A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: application to pharmacological MRI. Neuroimage, 32(2), 538-550.
9. Iliff, J. J., Wang, M., Liao, Y., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Science translational medicine, 4(147), 147ra111-147ra111.
Figures