3017
Effects of Tract Length in White Matter Alterations After Sports-Related Concussion: A Diffusion MRI Study from the NCAA-DoD CARE Consortium1Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 2Indiana University School of Public Health, Bloomington, IN, United States, 3Biostatistics, Indiana University School of Medicine, Indianapoilis, IN, United States, 4Medical College of Wisconsin, Milwaukee, WI, United States, 5University of California Los Angeles, Los Angeles, CA, United States, 6University of North Carolina, Chapel Hill, NC, United States, 7Virginia Tech University, Roanoke, VA, United States, 8University of Michigan, Ann Arbor, MI, United States, 9Psychiatry, Indiana University School of Medicine, Indianapolis, IN, United States
Synopsis
In the present study, we performed streamline tractography to characterize effects of tract length on white-matter microstructural alterations after sports-related concussion. Streamline length and counts were studied in affected white-matter fiber tracts that were found to have impaired white-matter integrity at some points along the tracts using voxel-based analyses. The results suggested that long fibers in the brains of collegiate athletes who sustained sports-related concussion are more vulnerable to this mild traumatic brain injury.
Purpose
Sport-related concussion (SRC) is an important public health issue. Since 2002, DTI has been used widely in neuroimaging studies of traumatic brain injury,1 resulting in more than 100 published studies.2-6 Although anatomical locations of significant white-mater alterations differ among studies, they have a common feature, i.e., belonging to long fiber tracts. White-matter abnormalities in fibers running in a dorsal-ventral orientation have been most commonly noted in the internal capsule (anterior and posterior limbs) and corona radiata (anterior, superior, and posterior).7-13 Abnormalities have also been reported in the corpus callosum,7,9,13,14 and the longitudinal fasciculus.8,10,12,13,15 While ROI- or voxel-based analyses are used in more than 90% of published diffusion MRI studies on SRC, tract-specific analyses have been less investigated. In this work, to characterize the susceptibility of fiber tracts to brain injury with respect to tract length, we performed streamline tractography and extracted tract-specific features including DTI metrics along the streamlines, normalized streamline counts, and streamline length. We investigated how these physical features of white-matter tracts affect their susceptibility to SRC and how these physical features modulate the clinical associations.Materials and Methods
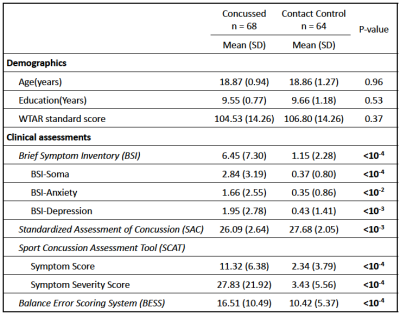
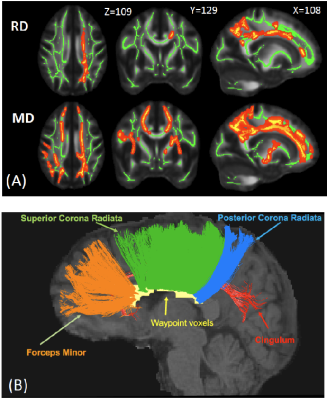
Participants: As part of the Concussion Assessment, Research and Education (CARE) consortium study on SRC, 68 collegiate athletes diagnosed with acute concussion (24-48 hours postinjury) and 64 matched contact-sport controls were included in this study (Table 1). The clinical assessments followed the protocol of the CARE Consortium study, which included a comprehensive SRC-specific battery of outcome measures.16 The control group, with matched sports type, number of prior concussions, and intellectual score (WTAR), received the same clinical assessments and multimodal MRI studies.17 DTI scans and parameters: DTI scans were performed in Siemens TimTrio and Prisma scanners across three study sites with an SS-SE-EPI sequence. The diffusion encoding scheme consisted 30 directions at b-value= 1000s/mm2 and 4 b0 volumes (b-value= 0s/mm2). One of the b0 volumes was acquired with a reversed phase-encoding direction. Other parameters included TE/TR= 98/7900ms, FOV= 243mm, matrix size= 90x90, whole brain coverage of 60 slices with a slice thickness of 2.7mm, and isotropic voxel at 2.7mm. Image processing: The diffusion-weighted images were de-noised using local PCA denoiser18 followed by motion, eddy, and fieldmap corrections using FSL EDDY_OPENMP19. DTI metrics were computed by FSL DITFIT. Tractography: Whole-brain white-mater streamline tractography was performed using CAMINO20 software with a q-ball and spherical harmonic reconstruction for fiber orientation distribution functions. To extract cortical-to-cortical connections, white matter and gray matter interface (derived from T1-weighted images) was used as starting and destination points for tractography with a step size of 0.5 and iterations of 25. Affected white-matter tracts were filtered by waypoint voxels that had significantly compromised white-matter integrity detected by DTI using tract-based spatial statistics (TBSS) analyses (Figure 1A, B). Data Analysis: The tract-specific features (i.e., length and normalized streamline count) were summarized from the affected tracts and the remain unaffected tracts (i.e., without waypoint filtering) for each subject. Paired t-tests, independent t-tests, and linear regression analyses were used for comparisons within a subject, between groups, and for correlations with clinical assessment scores, respectively.Results and Discussion
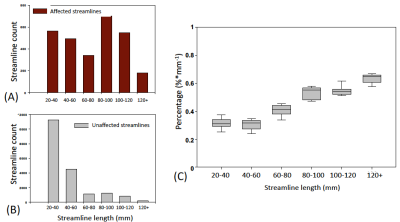
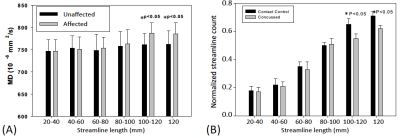
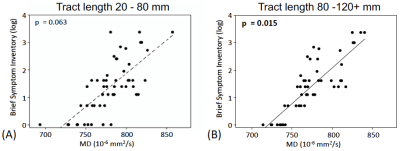
Four affected fiber tracts were identified: the forceps minor, superior and posterior corona radiata, and cingulum tracts (Figure 1B). Figure 2A, 2B show the length-wise distributions of streamlines for affected (A) and unaffected (B) tracts in one concussed athlete. Deriving (taking ratio) from Figure 2A and 2B for each concussed athlete, Figure 2C demonstrates that within the concussed group, longer tracts had a higher percentage of streamlines that were affected by SRC than shorter tracts. To correct for inherent bias from tract length itself, the original percentage was divided by the streamline length resulting in a length-adjusted percentage, %·mm-1. For a tract length longer than 80 mm, the length-adjusted percentage was higher than 0.5%·mm-1. Also, in the affected tracts, longer streamlines (>100mm) had significantly higher DTI mean diffusivity (MD) compared to the same length of streamlines in unaffected tracts in the same concussed subject (Figure 3A). Furthermore, the concussed athletes had significantly lower normalized streamline counts in longer tracts (>100mm) (Figure 3B). While shorter tracts (<80mm) demonstrated marginal significance, longer tracts (>80mm) presented significant correlations between MD and clinical assessment scores, suggesting the concussed athletes with higher MD in the affected white-matter tracts experienced worse psychological distress measured by Brief Symptom Inventory (BSI) (Figure 4B).Conclusion
In this study, we characterized the susceptibility of white-matter fiber tracts to SRC with respect to the tract physical features. The fiber tracts longer than 100mm appeared to be more vulnerable to this mild brain injury with higher MD, lower streamline counts, and significant clinical correlations.Acknowledgements
This publication was made possible, in part, with support from the Grand Alliance Concussion Assessment, Research, and Education (CARE) Consortium, funded, in part by the National Collegiate Athletic Association (NCAA) and the Department of Defense (DOD). The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Psychological Health and Traumatic Brain Injury Program under Award NO W81XWH-14-2-0151. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense (DHP funds.) Other funding supports include National Institutes of Health grant R21 NS075791 to YCW and TWM, R01 AG053993 to YCW.
The authors would also like to thank Jody Harland and Janetta Matesan (Indiana University); Ashley Rettmann (University of Michigan); Melissa Koschnitzke (Medical College of Wisconsin); Michael Jarrett, Vibeke Brinck and Bianca Byrne (Quesgen); Thomas Dompier, Melissa Niceley Baker, and Sara Dalton (Datalys Center for Sports Injury Research and Prevention); and the research and medical staff at each of the participating sites.
References
1. Arfanakis, K., V.M. Haughton, J.D. Carew, B.P. Rogers, R.J. Dempsey, and M.E. Meyerand, Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol, 2002;23(5):794-802.
2. Hulkower, M.B., D.B. Poliak, S.B. Rosenbaum, M.E. Zimmerman, and M.L. Lipton, A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol, 2013;34(11):2064-74.
3. Eierud, C., R.C. Craddock, S. Fletcher, M. Aulakh, B. King-Casas, D. Kuehl, and S.M. LaConte, Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin, 2014;4:283-94.
4. Gardner, A., F. Kay-Lambkin, P. Stanwell, J. Donnelly, W.H. Williams, A. Hiles, P. Schofield, C. Levi, and D.K. Jones, A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma, 2012;29(16):2521-38.
5. Dodd, A.B., K. Epstein, J.M. Ling, and A.R. Mayer, Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. J Neurotrauma, 2014;31(14):1235-48.
6. Shenton, M.E., H.M. Hamoda, J.S. Schneiderman, S. Bouix, O. Pasternak, Y. Rathi, M.A. Vu, M.P. Purohit, K. Helmer, I. Koerte, A.P. Lin, C.F. Westin, R. Kikinis, M. Kubicki, R.A. Stern, and R. Zafonte, A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav, 2012;6(2):137-92.
7. Zhang, L., L.A. Heier, R.D. Zimmerman, B. Jordan, and A.M. Ulug, Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol, 2006;27(9):2000-4.
8. Cubon, V.A., M. Putukian, C. Boyer, and A. Dettwiler, A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma, 2011;28(2):189-201.
9. Henry, L.C., J. Tremblay, S. Tremblay, A. Lee, C. Brun, N. Lepore, H. Theoret, D. Ellemberg, and M. Lassonde, Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma, 2011;28(10):2049-59.
10. Bazarian, J.J., T. Zhu, B. Blyth, A. Borrino, and J. Zhong, Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging, 2012;30(2):171-80.
11. Koerte, I.K., D. Kaufmann, E. Hartl, S. Bouix, O. Pasternak, M. Kubicki, A. Rauscher, D.K. Li, S.B. Dadachanji, J.A. Taunton, L.A. Forwell, A.M. Johnson, P.S. Echlin, and M.E. Shenton, A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus, 2012;33(6):E3: 1-7.
12. Murugavel, M., V. Cubon, M. Putukian, R. Echemendia, J. Cabrera, D. Osherson, and A. Dettwiler, A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. J Neurotrauma, 2014;31(22):1860-71.
13. Lancaster, M.A., D.V. Olson, M.A. McCrea, L.D. Nelson, A.A. LaRoche, and L.T. Muftuler, Acute white matter changes following sport-related concussion: A serial diffusion tensor and diffusion kurtosis tensor imaging study. Hum Brain Mapp, 2016;37(11):3821-3834.
14. McAllister, T.W., J.C. Ford, L.A. Flashman, A. Maerlender, R.M. Greenwald, J.G. Beckwith, R.P. Bolander, T.D. Tosteson, J.H. Turco, R. Raman, and S. Jain, Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology, 2014;82(1):63-9.
15. Meier, T.B., M. Bergamino, P.S. Bellgowan, T.K. Teague, J.M. Ling, A. Jeromin, and A.R. Mayer, Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum Brain Mapp, 2016;37(2):833-45.
16. Broglio, S.P., M. McCrea, T. McAllister, J. Harezlak, B. Katz, D. Hack, B. Hainline, and C.C. Investigators, A National Study on the Effects of Concussion in Collegiate Athletes and US Military Service Academy Members: The NCAA-DoD Concussion Assessment, Research and Education (CARE) Consortium Structure and Methods. Sports Med, 2017;47:1437-51.
17. Nencka, A.S., T.B. Meier, Y. Wang, L.T. Muftuler, Y.C. Wu, A.J. Saykin, J. Harezlak, M.A. Brooks, C.C. Giza, J. Difiori, K.M. Guskiewicz, J.P. Mihalik, S.M. LaConte, S.M. Duma, S. Broglio, T. McAllister, M.A. McCrea, and K.M. Koch, Stability of MRI metrics in the advanced research core of the NCAA-DoD concussion assessment, research and education (CARE) consortium. Brain Imaging Behav, 2018;12(4):1121-1140.
18. Manjon, J.V., P. Coupe, L. Concha, A. Buades, D.L. Collins, and M. Robles, Diffusion weighted image denoising using overcomplete local PCA. PLoS One, 2013;8(9):e73021.
19. Yamada, H., O. Abe, T. Shizukuishi, J. Kikuta, T. Shinozaki, K. Dezawa, A. Nagano, M. Matsuda, H. Haradome, and Y. Imamura, Efficacy of distortion correction on diffusion imaging: comparison of FSL eddy and eddy_correct using 30 and 60 directions diffusion encoding. PLoS One, 2014;9(11):e112411.
20. Cook, P.B., Y; Nedjati-Gilani, S; Seunarine, KK; Hall, MG; Parker, GJ; Alexander, DC. Camino: Open-Source Diffusion-MRI Reconstruction and Processing. in ISMRM. 2006. Seattle, WA, USA.
21. McCrory, P., W. Meeuwisse, J. Dvorak, M. Aubry, J. Bailes, S. Broglio, R.C. Cantu, D. Cassidy, R.J. Echemendia, R.J. Castellani, G.A. Davis, R. Ellenbogen, C. Emery, L. Engebretsen, N. Feddermann-Demont, C.C. Giza, K.M. Guskiewicz, S. Herring, G.L. Iverson, K.M. Johnston, J. Kissick, J. Kutcher, J.J. Leddy, D. Maddocks, M. Makdissi, G.T. Manley, M. McCrea, W.P. Meehan, S. Nagahiro, J. Patricios, M. Putukian, K.J. Schneider, A. Sills, C.H. Tator, M. Turner, and P.E. Vos, Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med, 2017;51(11):838-847.
22. Derogatis, L.R., BSI Brief Symptom Inventory: Administration, Scoring, and Procedure Manual (4th Ed.). 1993, Minneapolis, MN: National Computer Systems.
23. McCrea, M., J.P. Kelly, and C. Randolph, Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 1996, Waukesha, WI: CNS Inc.
24. Guskiewicz, K.M., S.E. Ross, and S.W. Marshall, Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train, 2001;36(3):263-273.
Figures