3016
Longitudinal White-Matter Abnormalities in Sports-Related Concussion: A Study of Diffusion Magnetic Resonance Imaging from the NCAA-DoD CARE Consortium1Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 2Indiana University School of Public Health, Bloomington, IN, United States, 3Psychiatry, Indiana University School of Medicine, Indianapolis, IN, United States, 4Biostatistics, Indiana University School of Medicine, Indianapoilis, IN, United States, 5Medical College of Wisconsin, Milwaukee, WI, United States, 6University of California Los Angeles, Los Angeles, CA, United States, 7University of North Carolina, Chapel Hill, NC, United States, 8Virginia Tech University, Roanoke, VA, United States, 9University of Michigan, Ann Arbor, MI, United States
Synopsis
In this study, we investigated the longitudinal recovery trajectories of white-matter microstructures in collegiate athletes who sustained sports-related concussion (SRC). We use diffusion tensor imaging (DTI) to detect white-matter alterations in collegiate athletes longitudinally at four timepoints: 24-48 hours postinjury, the point at which asymptomatic (cleared for return-to-play), seven days following return-to-play, and six months postinjury. We are interested in the extent of white-matter abnormalities over time and whether the white-matter changes persist beyond the point when athletes are considered clinical recovered (i.e., with normal clinical assessments).
Purpose
Sport-related concussion (SRC) is an important public health issue. While standardized assessment tools are useful in the clinical management of SRC,1 the underlying pathophysiology of SRC and the time course of pathophysiological recovery after injury remain unclear.2,3 We have previously shown that diffusion tensor imaging (DTI) is sensitive to acute changes in brain microstructures 24 to 48 hours post-SRC.4 In this study, we investigated the longitudinal recovery trajectories of white-matter microstructures in collegiate athletes who sustained SRC. Particularly, we are interested in the extent of white-matter abnormalities over time and whether the white-matter changes persist beyond the point when athletes are considered clinical recovered.Methods
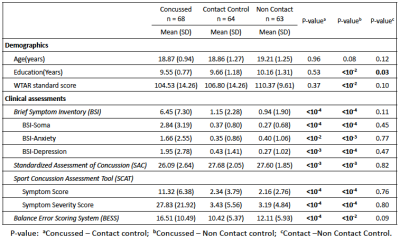
Participants: As part of the Concussion Assessment, Research and Education (CARE) consortium study on SRC, 68 collegiate athletes diagnosed with concussion, 64 matched contact-sport controls, and 63 matched non-contact-sport controls underwent clinical assessments and advanced MRI scans (Table 1). Clinical assessments followed the protocol of the CARE Consortium study and included a comprehensive SRC-specific battery of clinical outcome measures.5 The tests were performed on concussed athletes at four timepoints: 24-48 hours postinjury, the point at which asymptomatic (cleared for return-to-play), seven days following return-to-play, and six months postinjury. The two matched control groups, with matched sports type, number of prior concussions, and intellectual score (WTAR), received the same clinical assessments at similar timelines. All the participants underwent advanced MRI scans6 on the same day as clinical assessments. DTI scans and parameters: DTI scans were performed in Siemens TimTrio and Prisma scanners across three sites with an SS-SE-EPI sequence. The diffusion encoding scheme consisted 30 directions at b-value= 1000s/mm2 and 4 b0 volumes (b-value= 0s/mm2). One of the b0 volumes was acquired with a reversed phase-encoding direction. Other parameters included TE/TR= 98/7900ms, FOV= 243mm, matrix size= 90x90, whole brain coverage of 60 slices with a slice thickness of 2.7mm, and isotropic voxel at 2.7mm. Image processing: The diffusion-weighted images were de-noised using local PCA denoiser7 followed by motion, eddy, and fieldmap corrections using FSL EDDY_OPENMP8. DTI metrics computed by FSL DITFIT were non-linearly transformed to standard MNI space using ANTs registration9. Data Analysis: A common whole-brain while-matter skeleton map was extracted using FSL Tract-based spatial statistics (TBSS)10. TBSS was also used for voxelwise statistical analysis within the white-matter skeleton testing for group differences in diffusion metrics at each timepoint. Post-hoc ROI studies of TBSS significant voxels were used to demonstrate the longitudinal recovery of SRC, within-group correlation between DTI metrics with clinical assessment scores, and group-time interactions. Generalized-least-square models (GLS) adjusted for age, sites, and scanners was used for TBSS and ROI studies.Results
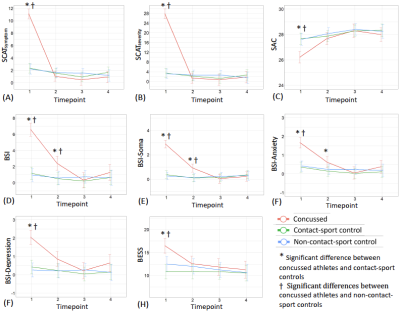
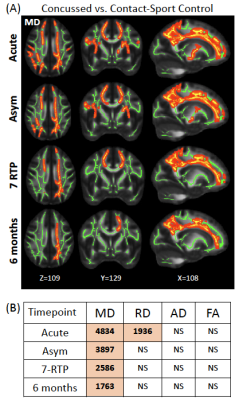
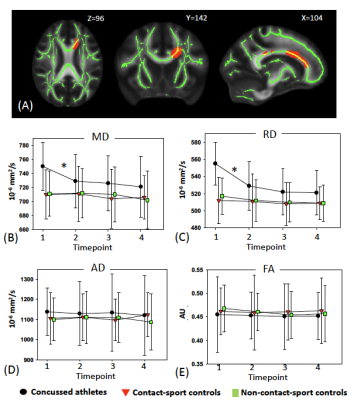
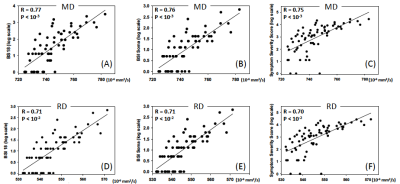
Table 1 summarizes demographics and clinical assessment scores at the acute timepoint. As expected, the concussed athletes differed significantly from the control groups at the acute timepoint for all the clinical assessments (Table 1 and Figure 1). Also, as expected, the acute changes in clinical outcomes recovered and there were no significant group differences at timepoint 2 (i.e., asymptomatic) and beyond, except for psychiatric distress measured by Brief Symptom Inventory (BSI) and its subcategories. DTI metrics in the brain white matter, however, demonstrated persistent group differences beyond the acute timepoint till 6 months postinjury. Particularly, radial diffusivity (RD) was significantly higher in the concussed athletes compared to the controls at the acute timepoint, and mean diffusivity (MD) was higher for all four timepoints (Figure 2(A)). The extent of white-matter abnormalities decreased over time as fewer voxels were detected significant in TBSS (Figure 2(B)). No differences in diffusion metrics were observed between the two control groups (contact- vs. non-contact-sport controls). White-matter areas with persistent group differences in DTI metrics (Figure 3(A)) were generated by intersecting all the TBSS significant voxels in Figure 2(A). The post-hoc ROI study of 512 persistent white-matte voxels demonstrated longitudinal recovery trajectories. MD and RD decreased significantly from the acute timepoint to a stable level, albeit still higher than controls, that lasted till 6 months postinjury (Figure 3(B,C)). In the persistently affected white matter, MD and RD increased with higher psychiatric distress level (BSI and BSI-soma) and symptom severity (Figure 4) at the acute timepoint.Discussion and conclusion
Consistent with previous small-scale studies,3,11-14 we found that changes in white-matter measured by DTI were detectable even when clinical assessments returned to normal levels. We further demonstrated that these significant changes, albeit decreasing in volume and intensity, persisted at 6 months postinjury, suggesting lasting effects of SRC. The group differences were mainly detected from concussion effects (concussed athletes vs. contact-sport controls), whereas there were no detectable differences from exposure effects (i.e., between the two control groups).Acknowledgements
This publication was made possible, in part, with support from the Grand Alliance Concussion Assessment, Research, and Education (CARE) Consortium, funded, in part by the National Collegiate Athletic Association (NCAA) and the Department of Defense (DOD). The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Psychological Health and Traumatic Brain Injury Program under Award NO W81XWH-14-2-0151. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense (DHP funds.) Other funding supports include National Institutes of Health grant R21 NS075791 to YCW and TWM, R01 AG053993 to YCW, P30 AG010133 and R01 AG019771 to AJS, and a Project Development Team within the ICTSI NIH/NCRR Grant Number UL1TR001108 to YCW.
The authors would also like to thank Jody Harland, Janetta Matesan (Indiana University); Ashley Rettmann (University of Michigan); Melissa Koschnitzke (Medical College of Wisconsin); Michael Jarrett, Vibeke Brinck and Bianca Byrne (Quesgen); Thomas Dompier, Melissa Niceley Baker, and Sara Dalton (Datalys Center for Sports Injury Research and Prevention); and the research and medical staff at each of the participating sites.
References
1. McCrea, M., K.M. Guskiewicz, S.W. Marshall, W. Barr, C. Randolph, R.C. Cantu, J.A. Onate, J. Yang, and J.P. Kelly, Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA, 2003;290(19):2556-63.
2. McCrory, P., W. Meeuwisse, J. Dvorak, M. Aubry, J. Bailes, S. Broglio, R.C. Cantu, D. Cassidy, R.J. Echemendia, R.J. Castellani, G.A. Davis, R. Ellenbogen, C. Emery, L. Engebretsen, N. Feddermann-Demont, C.C. Giza, K.M. Guskiewicz, S. Herring, G.L. Iverson, K.M. Johnston, J. Kissick, J. Kutcher, J.J. Leddy, D. Maddocks, M. Makdissi, G.T. Manley, M. McCrea, W.P. Meehan, S. Nagahiro, J. Patricios, M. Putukian, K.J. Schneider, A. Sills, C.H. Tator, M. Turner, and P.E. Vos, Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med, 2017;51(11):838-847.
3. Kamins, J., E. Bigler, T. Covassin, L. Henry, S. Kemp, J.J. Leddy, A. Mayer, M. McCrea, M. Prins, K.J. Schneider, T.C. Valovich McLeod, R. Zemek, and C.C. Giza, What is the physiological time to recovery after concussion? A systematic review. Br J Sports Med, 2017;51(12):935-940.
4. Mustafi, S.M., J. Harezlak, K.M. Koch, A.S. Nencka, T.B. Meier, C.C. Giza, J.P. DiFiori, K.M. Guskiewicz, J.P. Mihalik, S.M. LaConte, S. Duma, S.P. Broglio, A.J. Saykin, M. McCrea, T.W. McAllister, and Y.C. Wu, Acute White-Matter Abnormalities in Sports-Related Concussion: A Diffusion Tensor Imaging Study from the NCAA-DoD CARE Consortium. Journal of Neurotrauma 2018;35(22):2653-2664.
5. Broglio, S.P., M. McCrea, T. McAllister, J. Harezlak, B. Katz, D. Hack, B. Hainline, and C.C. Investigators, A National Study on the Effects of Concussion in Collegiate Athletes and US Military Service Academy Members: The NCAA-DoD Concussion Assessment, Research and Education (CARE) Consortium Structure and Methods. Sports Med, 2017;47:1437-51.
6. Nencka, A.S., T.B. Meier, Y. Wang, L.T. Muftuler, Y.C. Wu, A.J. Saykin, J. Harezlak, M.A. Brooks, C.C. Giza, J. Difiori, K.M. Guskiewicz, J.P. Mihalik, S.M. LaConte, S.M. Duma, S. Broglio, T. McAllister, M.A. McCrea, and K.M. Koch, Stability of MRI metrics in the advanced research core of the NCAA-DoD concussion assessment, research and education (CARE) consortium. Brain Imaging Behav, 2018;12(4):1121-1140.
7. Manjon, J.V., P. Coupe, L. Concha, A. Buades, D.L. Collins, and M. Robles, Diffusion weighted image denoising using overcomplete local PCA. PLoS One, 2013;8(9):e73021.
8. Yamada, H., O. Abe, T. Shizukuishi, J. Kikuta, T. Shinozaki, K. Dezawa, A. Nagano, M. Matsuda, H. Haradome, and Y. Imamura, Efficacy of distortion correction on diffusion imaging: comparison of FSL eddy and eddy_correct using 30 and 60 directions diffusion encoding. PLoS One, 2014;9(11):e112411.
9. Avants, B.B., N.J. Tustison, G. Song, P.A. Cook, A. Klein, and J.C. Gee, A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 2011;54(3):2033-44.
10. Smith, S.M., M. Jenkinson, H. Johansen-Berg, D. Rueckert, T.E. Nichols, C.E. Mackay, K.E. Watkins, O. Ciccarelli, M.Z. Cader, P.M. Matthews, and T.E. Behrens, Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 2006;31(4):1487-505.
11. Lancaster, M.A., T.B. Meier, D.V. Olson, M.A. McCrea, L.D. Nelson, and L.T. Muftuler, Chronic differences in white matter integrity following sport-related concussion as measured by diffusion MRI: 6-Month follow-up. Hum Brain Mapp, 2018.
12. Murugavel, M., V. Cubon, M. Putukian, R. Echemendia, J. Cabrera, D. Osherson, and A. Dettwiler, A longitudinal diffusion tensor imaging study assessing white matter fiber tracts after sports-related concussion. J Neurotrauma, 2014;31(22):1860-71.
13. Meier, T.B., M. Bergamino, P.S. Bellgowan, T.K. Teague, J.M. Ling, A. Jeromin, and A.R. Mayer, Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum Brain Mapp, 2016;37(2):833-45.
14. Henry, L.C., J. Tremblay, S. Tremblay, A. Lee, C. Brun, N. Lepore, H. Theoret, D. Ellemberg, and M. Lassonde, Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma, 2011;28(10):2049-59.
15. Derogatis, L.R., BSI Brief Symptom Inventory: Administration, Scoring, and Procedure Manual (4th Ed.). 1993, Minneapolis, MN: National Computer Systems.
16. McCrea, M., J.P. Kelly, and C. Randolph, Standardized Assessment of Concussion (SAC): Manual for Administration, Scoring and Interpretation. 1996, Waukesha, WI: CNS Inc.
17. Guskiewicz, K.M., S.E. Ross, and S.W. Marshall, Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train, 2001;36(3):263-273.
Figures