2989
Multimodal data revealed different biological substrates underlying intelligence for young males and females1Brainnetome Center, National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences, Beijing, China, 2University of Chinese Academy of Sciences, Beijing, China, 3the Mind Research Network, Albuquerque, NM, United States, 4Dept. of Psychiatry and Neurosciences, University of New Mexico, Albuquerque, NM, United States, 5Chinese Academy of Sciences Center for Excellence in Brain Science, Institute of Automation, Beijing, China
Synopsis
Individual differences in intelligence are usually measured using psychometric tests, which cover multifaceted cognitive domains and are strongly predictive of various life outcomes. Here, we employed connectome-based predictive modeling (CPM) to estimate individual’s IQ scores using either cortical thickness in grey matter or resting-state functional connectivity within fully cross-validations for males and females separately. Importantly, integrating multimodal neuroimaging data using CPM achieved improved prediction performance. Interestingly, we found that males and females use distinctively structured brains to achieve
Introduction
Individual differences in intelligence are usually measured using psychometric tests, which cover multifaceted cognitive domains including reasoning, processing speed, verbal comprehension, memory and spatial ability, and are strongly predictive of various important life outcomes1. Moreover, existing works on intelligence were limited to use a single modality, ignoring the complementary information provided by multimodal imaging data2. Consequently, we aim to quantitatively predict individuals’ IQ scores using multiple neuroimaging features for males and females separately (Fig 1).Methods
This analyses encompassed 326 healthy college students (age 19.0±1.1 years, 160 females/166 males). Full-Scale IQ scores were measured using the Chinese version of Wechsler Adult Intelligence Scale (WAIS-RC). Functional imaging data were preprocessed and paracellated into 116 nodes using the Automated Anatomical Labeling (AAL) 3 atlas, generating 6670 functional connectivity (FC) for each participant. Vertex-wise estimates of cortical thickness were calculated on a standardized cortical surface tessellation with 40962 vertices per hemisphere. We applied the connectome-based predictive modeling (CPM)4 to estimate individuals’ IQ scores within a leave-one-out-cross-validation for males and females separately (Fig 1). Specifically, each subject is designated as the test sample in turns while the remaining subjects are used to train the CPM model. First, FCs positively or negatively related to IQ scores with p values below a threshold across training participants were retained, constituting the high-IQ and low-IQ networks. Then, a summary statistic (network strength) was calculated by summing FCs in the high- or low-IQ network and entered into a simple linear regression model with the IQ scores respectively5. A general linear model was also constructed by combining the high- and low-IQ network strengths. Finally, FCs from the excluded testing subject was input into each predictive model, generating a predicted IQ score. This loop was repeated N (sample size) times to test through all subjects. The high- and low-IQ cortices were derived using features of cortical thickness in CPM. We also reran the CPM procedure by concatenating FC and cortical thickness horizontally as input features.Results
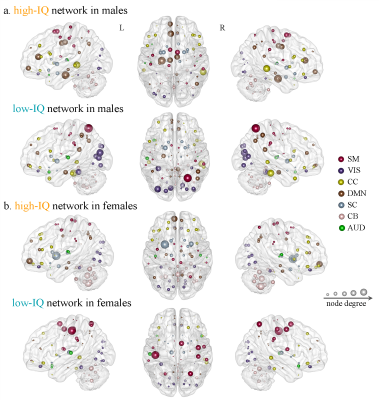
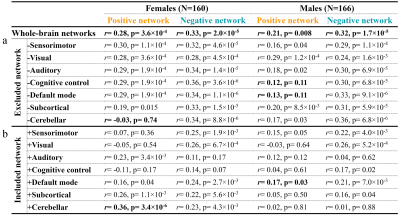
CPM achieved significant correlations between model-predicted and observed IQ scores, using whole-brain FCs, cortical thickness or their combination for males and females respectively, even after adjusting for age and mean frame-to-frame head motion (Fig 2a). No positively IQ-correlated features were detected in males. Combining FCs and cortical thickness yielded improved prediction performance (r[male] = 0.45, r[female] = 0.45, p<10-8; Fig 2b) than using any single modality alone. For males, the low-IQ cortex located exactly on the left inferior parietal lobule (IPL) and right precuneus (Fig 3a). For females, the high-IQ cortex concentrated on the right insula and right superior temporal gyrus; the low-IQ cortex distributed across the right IPL and right precuneus (Fig 3b). Regarding FCs, the high-IQ network exhibited dense connections in primarily default mode (DMN) and cognitive control (CC) networks for males (Fig 3c, 4a), and cerebellar network for females (Fig 3d, 4b). The low-IQ network exhibited diffuse connections across the brain, prominently in sensorimotor and visual networks for both males and females (Fig 4). Moreover, FCs of females in the high-IQ network were significantly longer in anatomical vector distance6 than these of males, while there is no difference in the low-IQ network between them (Fig 3e, f). Separately evaluating the importance of each canonical functional network defined by previous resting-state literature using CPM 7, we found that the cerebellar network demonstrated the most predictive power for females, while the network of DMN contributed mostly for males (Fig 5).Discussion
In current study, we accomplished successful prediction of IQ scores for males and females respectively, complementing existing works on individual differences in intelligence. Moreover, integrating multimodal neuroimaging features can yield improved prediction performance by capitalizing on the strength of each modality effectively2. Most importantly, we demonstrated that males and females use distinctive brain regions to achieve similar level of overall intelligence. Specifically, males’ intelligence demonstrated more closed correlations with grey matter cortex in left IPL and network in DMN, accounting for their superiority in spatial cognition and logical thinking8. In contrast, grey matter cortex in the right IPL and cerebellar network participated more in the processing of intelligence for females, coincident with their superiority in intuitive thinking, verbal learning, and item memory9. Promisingly, understanding the substrates of gender differences underlying intelligence may potentially promote personalized developmental cognitive programs and facilitate advancements in unbiased test design10. Furthermore, females and males can leverage their most efficient cognitive process in problem-solving, allowing more flexibility and positively impacting performance overall.Acknowledgements
This work is supported in part by China National Natural Science Foundation (No. 81471367, 61773380), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant No. XDBS01040100), the National Institute of Health (1R01EB005846, 1R56MH117107, 1R01MH094524, P20GM103472, P30GM122734) and the National Science Foundation (1539067).References
1. Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci 11, 201-211 (2010).
2. Sui J, et al. Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nature communications 9, 3028 (2018).
3. Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273-289 (2002).
4. Shen X, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protocols 12, 506-518 (2017).
5. Rosenberg MD, et al. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci 19, 165-171 (2016).
6. Dosenbach NU, et al. Prediction of individual brain maturity using fMRI. Science 329, 1358-1361 (2010).
7. Allen EA, et al. A baseline for the multivariate comparison of resting-state networks. Frontiers in systems neuroscience 5, 2 (2011).
8. Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci 17, 379-390 (2013).
9. Pezoulas VC, Zervakis M, Michelogiannis S, Klados MA. Resting-State Functional Connectivity and Network Analysis of Cerebellum with Respect to Crystallized IQ and Gender. Frontiers in human neuroscience 11, 189 (2017).
10. Hill AC, Laird AR, Robinson JL. Gender differences in working memory networks: a BrainMap meta-analysis. Biol Psychol 102, 18-29 (2014).
Figures