2883
Large field-of-view high resolution 3D imaging of the thoracolumbar nerve roots inside the spinal canal1University Medical Center Utrecht, Utrecht, Netherlands, 2Utrecht University, Utrecht, Netherlands, 3Philips Healthcare, Best, Netherlands, 4Swiss Federal Institute of Technology, Lausanne, Swaziland, 5Fondation Campus Biotech Geneva, Geneva, Swaziland, 6University Hospital Lausanne, Lausanne, Swaziland
Synopsis
A 3D T2/T1-weighted balanced-SSFP sequence has been developed to visualize the nerve roots inside the thoracolumbar spinal canal and their exit point from the spinal cord for clinical 3T MRI systems and coil setups. This sequence allows acquisition of large field-of-view, high resolution (sub-millimeter), almost isotropic images in less than 5 minutes. Furthermore, it is fully balanced, thus allowing high SNR, and intrinsically flow compensated. Hence, CSF flow artifacts are avoided. The acquired volumes in 15 volunteers showed good contrast between CSF, spinal cord and nerve roots (negative contrast), which could allow adequate visualization of intra-spinal pathologies.
Introduction
Magnetic resonance imaging (MRI) is the mainstay imaging technique for pathology within the spinal canal, including the spinal nerve roots [1-3]. However, the relatively low spatial resolution of most clinically used sequences hampers detection of subtle nerve root pathology (e.g. small tumors, traumatic edema) and visualization of individual nerve roots. Additionally, a small field of view (FOV) in the feet-head direction is usually imaged (standard FOV < 10 cm) to avoid unfeasible scan time. For long MRI acquisitions (> 5 minutes), small subject movements would corrupt the visualization of small structures like the spinal nerve roots. MR imaging of the nerve roots in the spinal canal is based on axial T2-weighted acquisitions [4].
The goal of this work is to develop an MRI sequence to visualize these nerve roots for the complete lumbar and large part of the thoracic spine. This sequence should provide high image contrast and high resolution in a clinically feasible acquisition time (5 minutes), and should be available for clinical MRI systems and coil setups.
Methods
A 3D balanced-Steady-State-Free-Precession (bSSFP) sequence was developed to visualize the nerve roots inside the spinal canal on a 3T Ingenia MRI scanner (Philips Healthcare, Best, NL) using the clinical anterior and posterior receive body coil arrays.
This sequence is T2/T1-weighted, thus the nerve roots appear with negative contrast against CSF (Fig.1,2). It has an almost isotropic resolution allowing multiplanar-reconstruction (MPR) with negligible loss of details (see sequence parameters, Fig.3). Furthermore, it is fully balanced, thus allowing high SNR, and intrinsically flow compensated. Hence, CSF flow artifacts affecting standard clinical sequences are avoided.
MR images were acquired on 15 volunteers, after obtaining written inform consent.
For comparison purposes, a clinically used axial 2D T2-weighted Turbo-Spin-Echo (TSE) sequence was acquired on one volunteer. Additionally, a clinically used axial 3D T2-weighted TSE sequence was acquired on another volunteer using a 3T Prisma MRI scanner (Siemens Healthcare, Erlangen, DE).
The image quality provided by the developed bSSFP sequence was evaluated for nerve root visualization and compared to the clinical 2D/3D T2-weighted TSE sequences.
Results
Fig.1 shows an example of the reproducibility of the sequence in 4 volunteers, as well as the high spatial resolution in both image planes (coronal and axial). Image acquisition was successful in all 15 volunteers.
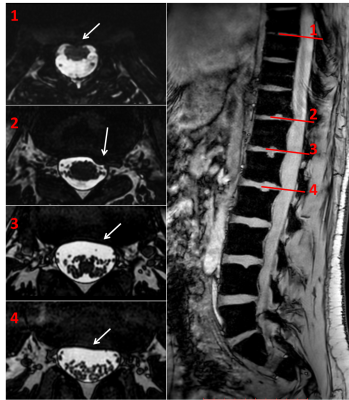
The large FOV in the feet-head direction enabled visualization of the complete lumbar spine as well as most of the thoracic spine (Fig.2). In addition, the high image contrast of the developed bSSFP sequence allowed for clear and sharp visualization of the nerve roots of the lower thoracic and lumbar spine and individual nerves of the cauda equina. However, imaging of the spinal vertebrae was limited due to the smaller FOV in the anterior-posterior direction.
The advantages of the increased spatial resolution and image contrast provided by the developed bSSFP sequence were also visible when comparing these images with the clinically used sequences (Fig.3).
These advantages are also illustrated in Fig.4 (movie), in which the acquired volume is displayed along the feet-head direction (axial MPR view) for one volunteer. The nerve roots and their exit points from the spinal cord can be clearly identified. Furthermore, ventral (motor) roots can be clearly distinguished from dorsal (sensory) roots, and a distinction can be made between nerve roots and vessels, which have a more tortuous course. However, it can also be observed that small banding artifacts (intrinsic in the bSSFP sequence) might sometimes be present at the extremities of the FOV in the feet-head direction.
Although patients were not included, one volunteer showed degenerative changes of the posterior lower thoracic and lumbar vertebrae that caused some bulging into the spinal canal (Fig.5). These degenerative changes were clearly visualized on the bSSFP sequence, including the relation to neighboring spinal nerve roots, suggesting that the increased spatial resolution and large FOV in the feet-head direction might aid in clinical decision-making.
Discussion and Conclusions
The developed 3D bSSFP sequence allows acquisition of high resolution (sub-millimeter), almost isotropic images of the thoracolumbar spine in less than 5 minutes acquisition time. The acquired bSSFP volumes showed good image contrast between CSF, spinal cord and nerve roots, which could allow adequate visualization of intra-spinal pathologies like (small) masses, e.g. meningiomas, schwannomas, cysts. Additionally, since this sequence allows visualizing where the nerve roots exit the spinal cord, it could become easier to predict whether spinal cord lesions (MS) will cause peripheral nerve issues, or explain why a lesion high in the spinal cord causes lower peripheral nerve issues. Moreover, this sequence can facilitate identification of the posterior roots innervating the leg muscles for targeted spinal cord stimulation [5]. Further validation in patients is warranted.Acknowledgements
No acknowledgement found.References
[1] Bertilson B., et al, BMC Musculoskeletal Disorders (2010) 11:202. DOI 10.1186/1471-2474-11-202.
[2] Gorbachova T., et al, Skeletal Radiol (2002) 31:511-515. DOI 10.1007/s00256-002-0508-x.
[3] Tawa N., et al, BMC Musculoskeletal Disorders (2016) 17:386. DOI 10.1186/s12891-016-1236-z.
[4] Hilal K., et al, Asian Spine Journal (2013) 7:184-189. DOI 10.4184/asj.2013.7.3.184.
[5] Wagner F., et al, Nature (2018) 563:65-71. DOI 10.1038/s41586-018-0649-2.
Figures