2869
Isotropic Restricted Diffusion Correlates with Tumor Cellularity in Pediatric and Adult Malignant Brain Tumors1Radiology, Washington University School of Medicine, Saint Louis, MO, United States, 2Medicine, University of Missouri – Kansas City, Kansas City, MO, United States, 3Medical Scientist Training Program, The University of Alabama at Birmingham, Birmingham, AL, United States, 4Radiology, The First Affiliated Hospital of Nanchang University, Nanchang, China, 5Radiology, Guangzhou First People’s Hospital, Guangzhou, China, 6Pediatrics, Washington University School of Medicine, Saint Louis, MO, United States, 7Pathology and Immunology, Washington University School of Medicine, Saint Louis, MO, United States
Synopsis
We demonstrated how ADC inconsistently correlates with histologically determined tumor cellularity rendering it an unreliable imaging biomarker of tumor cellularity in pediatric and adult malignant brain tumors. In contrast, modified-diffusion basis spectrum imaging (DBSI)-derived restricted isotropic diffusion fraction correlated with histology-determined cellularity in pediatric and adult malignant brain tumors.
Introduction
Pediatric brain tumors, different from those of adults in both genetic and phenotypic profiles, are the leading cause of cancer-related death in children in the United States. Clinically, Gd-enhanced T1-weighted image (Gd-T1W) and fluid attenuated inversion recovery (FLAIR) T2-weighted image are the standard imaging sequences to assess brain tumor. Typically, malignant brain tumor is identified by increased tumor cellularity. Tumors of increased cellularity would require surgical intervention. Diffusion MRI-derived ADC is one of the most widely employed imaging metrics to detect increased brain tumor cellularity,1 manifesting as an inversed relationship with tumor cellularity. In cases of high-grade gliomas, ADC often fails to accurately reflect tumor cellularity because high-grade gliomas consist of tumor necrosis, microvascular proliferation, and cellular infiltration impacting ADC differently, some contradictorily. We recently modified diffusion basis spectrum imaging (DBSI)2 to characterize pathology-induced structural changes in brain tumor tissues. Specifically, modified-DBSI-derived restricted isotropic diffusion tensor fraction (restricted fraction) reflects tumor cellularity. To determine whether modified-DBSI derived restricted fraction is a biomarker of tumor cellularity for various malignant brain tumors, we examined seven brain tumor specimens from six autopsied brain specimens.Materials and Methods
Seven brain tumor specimens from six autopsied brains were resected and immediately fixed in 10% formalin. Specimens were scanned using a 4.7T Agilent small animal MR scanner and a surface coil. A spin-echo diffusion-weighted sequence with 99-diffusion-encoding directions and maximum b-value=3000 s/mm2 was employed to acquire DW images with the following imaging parameters: TR=1500 ms, TE=40 ms, slice thickness=0.5 mm, field-of-view=2.4 × 2.4 cm2, matrix size=96 x 96. T1W images were acquired using a gradient echo sequence with TR=80 ms and TE=10 ms. T2W images were acquired using a spin echo sequence with TR=4000 ms and TE=40 ms. Tissues were sectioned for H&E staining. Histology slides were digitized using a NanoZoomer 2.0-HT System (Hamamatsu, Japan) with a 20× objective for analyses. All histological slides were reviewed by an experienced neuropathologist.Results
Gd-T1W image displayed a large enhancing lesion in the right posterior region of a 16-year-old pediatric patient with pathologically confirmed primitive neuroectodermal tumor (PNET, WHO Grade IV) and diffuse astrocytoma (WHO Grade II) (Fig. 1A). Corresponding postmortem autopsy mass effect was also seen in the specimen (Fig. 1B). We dissected and scanned two tissue blocks from the specimen (Fig. 1B, red dotted squares). Modified-DBSI restricted fraction correlated with the distributions of high-grade tumor cellularity (black dash outlines on H&E images) in both cases (Fig. 1C, D). The regions of decreased ADC (Fig. 1C, D, arrows) appeared to align with low grade tumor (red dash outlines on H&E images) and hemorrhage regions.
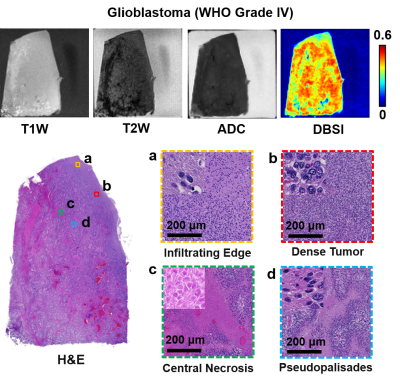
Brain autopsy of a separate pediatric case of an 8-year-old patient with polyphenotypic brain tumor showed a mass in the brain stem and cerebellum region (Fig. 2A). Intense signals from modified-DBSI restricted fraction highlighting similar areas in H&E imaging (Fig. 2C, black outline) that indicated increased cellularity. The regional ADC increased, contrary to expectation. In the case of an adult patient with glioblastoma (GBM, WHO Grade IV), H&E imaging of the tissue showed typical heterogeneous tumor pathologies, including tumor infiltration (Fig. 3, yellow squares), sheet-growth tumor cells (Fig. 3, red squares), central necrosis (Fig. 3, green squares), and pesudopalisides (Fig. 3, blue squares). Low intensity in T2W image indicated central necrosis and hemorrhage regions. T1W image and ADC map displayed insignificant signal contrast but modified-DBSI restricted fraction showed heterogeneous signal distribution. The modified-DBSI restricted fraction hyperintensity correlated with increased tumor cellularity while the hypointensity matched necrosis and hemorrhage regions.
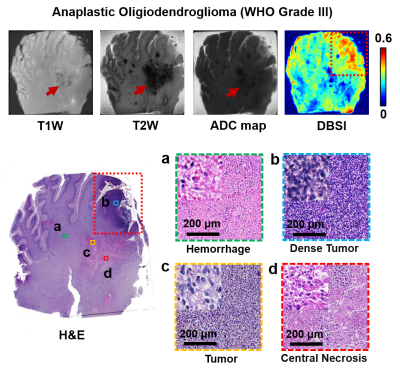
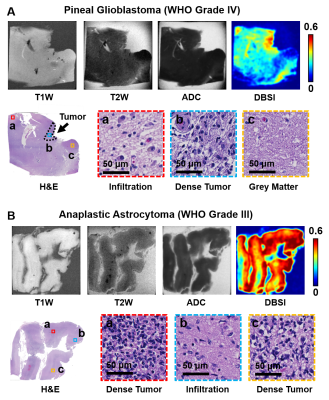
In a specimen from an 18-year-old patient with pediatric anaplastic oligodendroglioma (WHO Grade III), T1W image, T2W image, and ADC map all displayed contrast between the lesion (Fig. 4, red arrows) and surrounding tissues, which contained necrosis and hemorrhage areas according to H&E findings. Modified-DBSI restricted fraction correctly located dense tumor region (Fig. 4, yellow arrows) as indicated by histological staining. Restricted fraction also showed hypointensity in the necrosis and hemorrhage region. Lastly, T1W, T2W, and ADC showed little or no image contrast in specimens from two separate pediatric patients with pineal GBM (WHO Grade IV, Fig. 5A) and anaplastic astrocytoma (WHO Grade III, Fig. 5B), respectively. Modified-DBSI clearly revealed tumor cellularity distribution in agreement with H&E staining results in both patients.
Discussions and Conclusions
We have demonstrated how ADC inconsistently correlates with histologically determined tumor cellularity rendering it an unreliable imaging biomarker of tumor cellularity in pediatric and adult malignant brain tumors. Contrary to ADC, modified-DBSI restricted fraction significantly correlated with tumor cellularity, demonstrating the potential of restricted fraction as a promising biomarker for assessing high-grade glioma.Acknowledgements
This work was supported in part by NIH R01-NS047592, P01-NS059560, U01-EY025500, National Multiple Sclerosis Society (NMSS) RG 5258-A-5, RG 1701-26617, and Department of Defense Idea Award W81XWH-12-1-0457, The Taylor Rozier Hope for a Cure Foundation, Fundamental Research Funds for the Central Universities, SCUT (2018MS23), and Natural Science Foundation of Guangdong Province in China (2018A030313282).References
1. Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. Jun 2005;235(3):985-991.
2. Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain : a journal of neurology. Dec 2011;134(Pt 12):3590-3601.
Figures