2731
Differential Middle Cerebral Artery Plaque Characteristics in Patients with Transient Ischemic Attack and Ischemic Stroke: A High-Resolution MR Vessel Wall Imaging Study1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, los angeles, CA, United States, 2Radiology, Xuanwu Hospital Capital Medical University, Beijing, China, 3Neurology, Beijing Chaoyang Hospital, Capital Medical university, Beijing, China, 4Radiology, Beijing Chaoyang Hospital, Capital Medical university, Beijing, China
Synopsis
This study is to compare the characteristics of intracranial plaques between TIA and stroke patients using VWI. Sixty-two patients (31 TIA and 31 stroke) with MCA stenosis were enrolled in the study. Routine brain MRI, TOF-MRA, pre and post- contrast VWI were performed on each patient. Morphological features of the culprit plaque were compared between the two groups. TIA group had a lower occurrence of hyperintensity plaque, plaque surface irregularity and enhancement grade, those features showed no statistically significant differences and also the degree of stenosis and RI. VWI is useful modality for assessing the intracranial plaques in TIA patients.
Introduction
Intracranial atherosclerotic disease (ICAD) is a major cause of transient ischemic attack (TIA).1 It is essential to diagnose TIA promptly because of its associated high risk for more devastating vascular event - ischemic stroke.2 However, this is challenged by the lack of definite infarction and short duration of symptoms. The current routine imaging workup is also limited in the evaluation of treatment efficacy. Vessel wall imaging (VWI) is emerging as a non-invasive imaging modality for directly assessing ICAD lesions.3 The aim of this study is to image the intracranial vessel wall in TIA patients using VWI and demonstrate that certain TIA patients have similar plaque characteristics with stroke patients, which can be used to diagnose TIA patients with high risk for stroke.Methods
We retrospectively enrolled 62 atherosclerotic patients (31 TIA patients and 31 stroke patients) from our institutional VWI database. The inclusion and exclusion criteria are shown in Table 1. All scans were obtained on a 3T system (MAGNETOM Prisma, Siemens, Erlangen, Germany) equipped with a 64-channel head-neck coil. The protocol included routine brain MRI (T1-, T2-weighted, FLAIR, and DWI), 3D TOF-MRA, pre- and post-contrast VWI. VWI was performed using a recently developed 3D whole-brain VWI sequence4,5 with following parameters: TR/TE = 900/15 ms; field of view = 173×210 mm2; voxel size = 0.55×0.55×0.55 mm3; scan time = 8 minutes. Post-contrast VWI was acquired 5 minutes after a bolus injection of Gd-DTPA at a dose of 0.2 mmol/kg body weight. VWI images were compared between the two patient groups with respect to the plaque signal intensity, enhancement pattern, plaque surface irregularity, degree of stenosis and remodeling index (RI).6 Categorical variables were analyzed using a χ2 test, and continuous variables were compared using t test or Mann–Whitney U test between the two groups. Values of p< 0.05 were considered statistically significant.Results
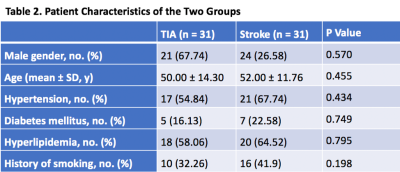
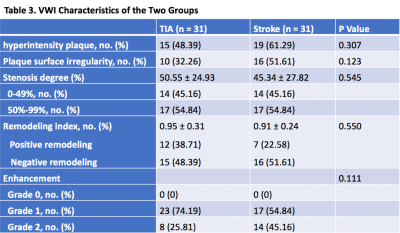
There were no significant differences in gender (p= 0.57), age (p= 0.46), risk factors (hypertension, diabetes mellitus, hyperlipidemia), and history of smoking (p=0.198) (Table 2). On VWI, eccentric wall-thickening at the MCA stenosis, which suggests atherosclerotic plaque,7 was observed in all patients. Fifteen (48.39%) patients in the TIA group were found to have hyperintensity plaques and 19 (61.29%) in the stroke group. The plaque surface irregularity in the TIA group was 10 (32.26%), compared with 16 (51.61%) in the stroke group. The stenosis degree of both groups was also estimated on VWI, showing the same classification to MRA (i.e. 0-49% in 14 patients (45.16%), and 50%-99% in 17 patients (54.84%)). The occurrence of positive/negative remodeling is 12 (38.71%)/15 (48.39%) in TIA group, and 7 (22.58%)/16 (51.61%) in stroke group. All 31 plaques in TIA group enhanced (grade 1, 74.19%; grade 2, 25.81%), and all 31 plaques in stroke group enhanced (grade 1, 54.84%; grade 45.16%). The occurrence of hyperintensity plaque, plaque surface irregularity and the grade of plaque enhancement in the stroke group were slightly higher than in the TIA group. However, there were no statistically significant differences between the two groups in terms of these characteristics (Table 3). Representative TIA and ischemic stroke cases are shown in Figure 1 and 2, respectively.Discussion
In our study, 14 TIA patients (45.16%) had only mild stenosis on MRA. Through MR VWI, intracranial atherosclerotic plaques with vulnerable features were also been detected in these patients with mild stenosis, which provided valuable information for identifying the culprit lesions. For the mechanisms of TIA, there still remain several hypotheses including micro-emboli, hemodynamic impairment, vasospasm and so on. If the border zone or collateral circulation of the remote cortical branch is still normal, or the number of micro-emboli formation is less, which does not go beyond the threshold of brain tissue scavenging micro-emboli, it can only represent as TIA.8 Patients in TIA group had the similar characteristics of plaque as the stroke group in our study. Therefore, we speculate that the mechanism of washing out microemboli in these patients works well temporarily and the amount of microemboli has not exceed the threshold of washing out the microemboli in brain tissue. However, the presence of these vulnerable plaques remains a risk factor for recurrent TIA and stroke.Conclusion
MR VWI can be used to identify vulnerable plaques in patients with TIA and to provide evidence for clinical decision and risk-stratification. Further prospective studies are required to illustrate the relationship between vulnerable plaque and the incidence and prognosis of TIA.Acknowledgements
Thanks to all radiologists, MRI technicians and neurologists who participated in this study in Cedars Sinai Medical Center, Beijing Chaoyang Hospital and Xuanwu Hospital, for their contributions to the study.References
1. Battistella V, Elkind M. Intracranial atherosclerotic disease[J]. Eur J Neurol, 2014,21(7):956-962.
2. Nadarajan V, Perry RJ, Johnson J, et al. Transient ischemic attacks: mimics and chameleons. Pract Neurol. 2014 Feb;14(1):23-31.
3. Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2017;38(2):218–29.
4. Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magn Reson Med 2017; 77:1142-50.
5. F Wu, Q Ma, H Song, et al. Differential Features of Culprit Intracranial Atherosclerotic Lesions: A Whole‐Brain Vessel Wall Imaging Study in Patients with Acute Ischemic Stroke. J Am Heart Assoc. 2018 Jul 22;7(15).
6. Wu F, Song H, Ma Q, et al. Hyperintense Plaque on Intracranial Vessel Wall Magnetic Resonance Imaging as a Predictor of Artery-to-Artery Embolic Infarction. Stroke. 2018 Apr;49(4):905-911.
7. Qiao Y, Zeiler S R, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014,271(2):534-542.
8. Yu Y P, Tan L. The Vulnerability of Vessels Involved in the Role of Embolism and Hypoperfusion in the Mechanisms of Ischemic Cerebrovascular Diseases. Biomed Res Int, 2016,2016:8531958.
Figures

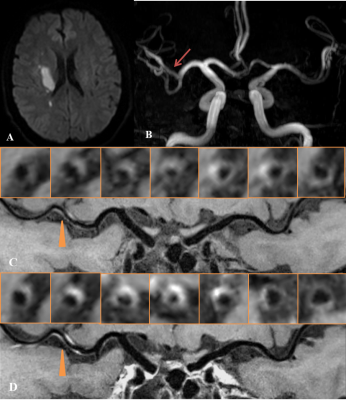
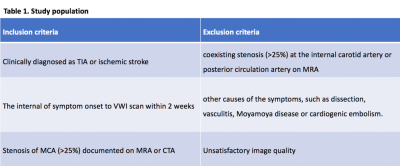
Table 1. The inclusion and exclusion criteria.