2576
Olfactory bulb atrophy and smell deficits in H&Y stage-1 Parkinson’s disease1Radiology, Penn State University, Hershey, PA, United States, 2Neurology, Penn State University, Hershey, PA, United States
Synopsis
The olfactory bulb (OB) is highly affected by Lewy bodies, the hallmark pathology of Parkinson's disease (PD). Hyposmia has been reported to occur in the majority of early-stage PD patients. We investigated whether there is olfactory bulb atrophy in early-stage PD patients. Our data demonstrated significantly reduced olfactory bulb volumes as well as lower psychophysical smell test scores in H&Y stage-1 PD patients compared to healthy controls.
Introduction
Parkinson’s disease (PD) is a devastating neurodegenerative disease characterized by motor and non-motor symptoms. Hyposmia has been reported to occur in the majority of early-stage PD patients, with deficits in odor detection, identification, and discrimination 1-3. Postmortem studies show that olfactory bulb (OB) is highly affected by Lewy bodies. However, whether there are olfactory bulb morphological changes in early-stage PD is not clear. We hypothesize that there will be detectable atrophy in the OBs of PD patients relative to healthy controls (HCs). In this study, we investigated differences in olfactory bulb volume and psychophysical tests of smell function in PD and HCs.Methods
Human Subjects
Seventeen idiopathic stage 1 PD participants and 14 age/sex-matched HCs were included in this study. There were no significant differences in age, sex, education, or cognitive scores between the two groups (Table 1). All subjects gave written informed consent, which was approved by the local institutional review board.
Data Acquisition
Olfactory functions were assessed with psychophysical tests. Smell identification function was evaluated using either the 40-item University of Pennsylvania Smell Identification Test (UPSIT) or the 20-item OLFACT-C Olfactory Identification Test (Osmic Enterprises, Inc.). Smell detection thresholds were obtained using the OLFACT-C Olfactory Threshold Test (Osmic Enterprises, Inc.). For each test, each nostril was assessed separately by blocking one nostril with tape. The MRI study was conducted on a Siemens 3 T scanner with a 64-channel head/neck coil. The morphological imaging of the brain was acquired with a T1-weighted MPRAGE method in 1-mm3 resolution with TR/TE/FA = 2300 ms/ 2.98 ms/ 9°. The morphological MRI for the OB was conducted with a T2-weighted 3D ZOOMit method: TR/TE/FA=2600 ms / 77 ms /120°, FOV=164 mm, acquisition matrix = 512´158´80, image resolution=0.3 mm´0.3 mm´0.5 mm, average=4.
Data Processing and Analysis
Left and right OB volumes were measured via manual segmentation of two raters blinded to patient status and olfactory scores (Figure 1). Raters achieved left and right interclass correlations of 0.95 and 0.97, respectively. Intracranial volume (ICV) was calculated using the sum of the separate segmentations of grey matter, white matter, and cerebrospinal fluid in SPM. Analysis of OB volume was performed using absolute volumes as well as ICV normalized volumes. ICV normalized OB volumes were calculated by (OB volume / ICV) * average ICV of both groups. Statistical values for all analysis were computed using SPSS software version25 (SPSS Inc., Chicago, IL, USA ) corrected for multiple comparisons, Bonferonni when appropriate.
Results
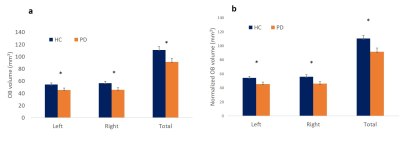
Psychophysical test scores of smell functions were significantly lower in early-stage PD patients compared to that of HCs (p < 0.001) (Figure 2). Comparison of olfactory bulb volumes showed significant differences between the two groups when using absolute OB volume values (left: p = 0.036, right: p = 0.014, total: p = 0.014) and ICV normalized volumes (left: p = 0.038, right p= 0.019, total: p = 0.014) (Figure 3). There were no significant differences between left and right sides in smell functions or OB volumes in either group.Conclusions
Results from the smell threshold and identification tests show significant smell deficits in early-stage PD patients. In alignment with reduced smell functions in PD patients, OB volume was found to be reduced in the PD group. Our findings support our hypothesis that there is atrophy of the OB in early stage PD. To further validate this finding, longitudinal studies are necessary.Acknowledgements
This study was supported by the NIH grant R01NS099630 and the Department of Radiology, Pennsylvania State University College of Medicine.References
[1]. Haehner A, et al., Parkinsonism Relat Disord, 2009. 15: 490.
[2]. Muller A, et al., J Clin Neurosci, 2002. 9: 521.
[3]. Doty RL, et al., Neurology, 1988. 38: 1237.
Figures