2505
NOE enhancement for 31P-MRS of skeletal and cardiac muscle at 7T1Institute of Neuroscience and Medicine-4, Forschungszentrum Juelich, Juelich, Germany, 2Oxford Centre for Clinical Magnetic Resonance Research (OCMR), University of Oxford, John Radcliffe Hospital, Oxford, United Kingdom, 3Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislave, Slovakia, 4Wellcome Centre for Integrative Neuroimaging, FMRIB, University of Oxford, John Radcliffe Hospital, Oxford, United Kingdom, 5High-Field MR Centre, Centre for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 6Institute of Neuroscience and Medicine-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 7JARA-BRAIN-Translational Medicine, Aachen, Germany, 8Department of Neurology, RWTH Aachen University, Aachen, Germany, 9The Wolfson Brain Imaging Centre, University of Cambridge, Cambridge Biomedical Campus, Cambridge, United Kingdom
Synopsis
Phosphorus MR spectroscopy (31P-MRS) is a proven method for probing tissue energetics and membrane metabolism. Nuclear Overhauser effect (NOE) enhancement can considerably improve the quality of 31P spectra. This has been demonstrated in the brain and prostate at 7T, but NOE has not yet been applied elsewhere in the human body at 7T. In this study, we evaluated NOE enhancement for 31P-MRS in human skeletal muscle and in the heart in vivo at 7T. We observe significant enhancements (e.g. for PCr/γ-ATP: 25%/16% in muscle, 31%/11% in
Introduction
In vivo phosphorus MR spectroscopy (31P-MRS) has been used for several decades as a tool to non-invasively assess tissue energetics and membrane metabolism1. Cardiac 31P-MRS is of particular interest as the impairment of cardiac energy metabolism is indicative of various cardiac disorders2. The inherently low signal-to-noise ratio (SNR) of 31P-MRS can be mitigated by using ultra-high fields, e.g., 7T3. To further boost the SNR of high-energy metabolites, nuclear Overhauser effect (NOE) enhancement is frequently employed at 1.5T and 3T. This method has been previously demonstrated in the brain4,5 and prostate6, but has not yet been applied to skeletal and cardiac muscle tissues at 7T.
In this study, we aimed to evaluate the NOE enhancement of 31P-MRS at 7T in human thigh muscle and in the heart in vivo.
Material and methods
Ten healthy volunteers (3F/7M, mean age 32 ± 8 years, BMI = 24.1 ± 4.2 kg.m-2) were recruited for this study, and scanned supine in a whole-body 7T MR system (Siemens, Erlangen, Germany), after informed consent was obtained in compliance with ethical and legal requirements. A double-tuned, purpose-built Tx/Rx coil, consisting of a pair of 1H-trapped 15 cm 31P loops driven in quadrature, and a single 10 cm 1H loop7 was positioned over the volume of interests, i.e. over the thigh muscle in five volunteers and over the heart in the other five.
Figure 1 shows the sequence diagram used in this study. 31P MR spectra were obtained using an acquisition weighted 3D UTE-CSI pulse sequence8 with scan parameters of 16×8×8 matrix, 240×240×200 mm3 field-of-view and 4 averages at the centre of the k-space. The TR was set to 1.5 s for muscle and 1 s for the heart examinations, as the T1 relaxation times are shorter in the heart. NOE was applied as a 1H pulse train at the maximum allowed voltage consisting of 10×10 ms long rectangular pulses (short NOE), or of 8×100 ms long pulses filling the rest of the TR (long NOE). An acquisition without the introduction of NOE pulses (no NOE) was also acquired as a reference. In the case of skeletal muscle, only the short NOE version was applied.
Three voxels in either deep-lying thigh muscle or in the mid-septum were selected from the WSVD9 combined data for analysis using the OXSA toolbox10. Due to the limited bandwidth of the excitation pulse, β-ATP was at the edge of the excitation bandwidth, and thus not analysed. The NOE enhancement was quantified as the percentage increase in the fitted 31P signals against the reference (without NOE).
Results
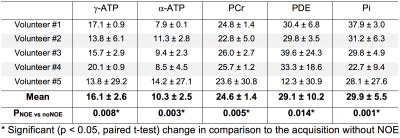
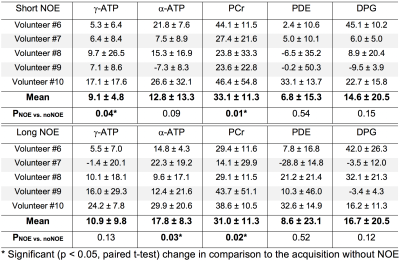
Examples of 31P spectra obtained with and without NOE enhancement are shown in the selected region in skeletal muscle (Fig. 2) and heart (Fig. 3). Tables 1 and 2 summarise the effect of NOE on important 31P metabolites in skeletal muscle and the heart respectively. Although a negative effect was observed in some cardiac metabolites across volunteers (Table 2), the average increase is positive for all metabolites analysed. There were no statistically significant differences (p > 0.05, paired t-test) found between the effects of long NOE and short NOE protocols on cardiac energy metabolites.Discussion
We have demonstrated the feasibility of using the NOE technique to enhance signal intensity of high-energy metabolites in human skeletal muscle and heart in healthy volunteers at 7T. The NOE signal enhancement for PCr observed in this study is similar between skeletal and cardiac muscle, i.e. 33.1 ± 11.3% vs. 24.6 ± 1.4%; these values are comparable to 7T reports from brain 24.3 ± 1.6%4,5 and prostate 20.0 ± 17.0%6. Similarities of enhancement in γ- and α-ATP between skeletal and cardiac tissue were also found, i.e. 9.1 ± 4.8% vs. 16.1 ± 2.6% and 12.8 ± 13.3% vs. 10.3 ± 2.5%, respectively. Overall, the signal intensities of all detected 31P metabolites increased after applying the NOE pulses, thus improving the SNR of the acquired data in both skeletal muscle and the heart. This is in agreement with previous examinations performed in the human brain4,5 and the prostate6 at 7T. Even though a small negative NOE effect was reported for γ- and α-ATP in the prostate6, other metabolites were enhanced positively. Potential limitations employing the NOE technique would be associated with increased SAR and timing constraints in sequences. However, this study was carried out within regulatory SAR limits and without increasing the scan duration.Conclusions
The use of NOE at 7T leads to a 25% and 31% increase in signal intensity in human skeletal and cardiac muscle, respectively. This increase in SNR, and hence in fitting accuracy, can significantly improve the 31P-MRS quality in future cardiac metabolism studies at 7T.Acknowledgements
Funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society to CTR [098436/Z/12/B]; and an FZJ international travel grant to CHC. The Austrian Science Fund FWF [J 4043] and the Slovak Grant Agencies VEGA [2/0001/17] and APVV [15-0029] are also acknowledged.References
1. Valkovic L, et al. In-vivo 31P-MRS of skeletal muscle and liver: a way for non-invasive assessment of their metabolism. Analytical Biochemistry. 2017, 529:193.
2. Bottomley PA, NMR Spectroscopy of the human heart. In: Harris RK, Wasylishen RE, editors. Encyclopedia of magnetic resonance. Chichester: John Wiley; 2009.
3. Rodgers CT, et al. Human cardiac 31P magnetic resonance spectroscopy at 7 tesla. MRM. 2014, 72:304.
4. Lagemaat MW, van de Bank BL et al. Repeatability of 31P MRSI in the human brain at 7T with and without the nuclear Overhauser effect. NMR Biomed. 2016;29:256-263.
5. Lei H, et al. In vivo 31P magnetic resonance spectroscopy of human brain at 7T: An initial experience. MRM. 2003, 49:199.
6. Lagemaat MW et al., 31P MR spectroscopic imaging of the human prostate at 7T: T1 relaxation times, nuclear Overhauser effect, and spectral characterization. MRM. 2015, 73:909.
7. Valkovic L, et al. Adiabatic excitation for 31P MR spectroscopy in human heart at 7T: A feasibility study. MRM. 2017, 78:1667.
8. Robson MD, et al. Ultrashort TE chemical shift imaging (UTE-CSI). MRM. 2005, 53:267.
9. Rodgers CT and Robson MD. Receive array magnetic resonance spectroscopy: Whitened singular value decomposition (WSVD) gives optimal Bayesian solution. MRM. 2010, 63:881.
10. Purvis LAB, et al. OXSA: an open-source magnetic resonance spectroscopy analysis toolbox in MATLAB. PLoS ONE. 2017, 12(9):e0185356.
Figures