2324
Golden angle radial undersampling to accelerate synthetic CT generation with generative adversarial networks for prostate MR-guided Radiotherapy1Radiotherapy, UMC Utrecht, Utrecht, Netherlands, 2Center for Image Sciences, UMC Utrecht, Utrecht, Netherlands, 3Image Science Institute, UMC Utrecht, Utrecht, Netherlands
Synopsis
Synthetic-computed tomography (sCT) is crucial to enable MR-only radiotherapy and accurate MR-based dose calculations. In this work, we assessed the feasibility of using undersampled golden angle radial acquisition in combination with a conditional adversarial network to accelerate both acquisition and sCT generation for patients with prostate cancer. Golden angle radial acquisitions were simulated for several undersampling factors in a retrospective manner on 3D Cartesian spoiled gradient-echo data that were clinically acquired on
Purpose
Synthetic-computed tomography (sCT) images are crucial to enable MR-only Radiotherapy (RT) and accurate MR-based dose calculations1. For MR-guided RT, MR acquisition and sCT generation should be fast (<1min) to facilitate online dose calculations and adaptations of the treatment. Previously, we demonstrated that sCT generation could be approached with deep learning as an image-to-image translation problem by generative adversarial networks (GANs) for general pelvis MR-only RT2. However, image acquisition for sCT generation still requires between 2-10min for large FOV and high resolution1. In general, undersampled GA radial acquisition in combination with compressed sensing can meet these criteria. However, this requires longer reconstruction times3. Here, we propose using golden angle (GA) radial undersampling schemes4 in combination with a GAN-based method to generate sCT as a mean to accelerate MRI acquisition5 and sCT generation2. Particularly, we investigate the capability of GANs in solving image-to-image translation in the presence of aliasing artefacts.Material & Methods
A study was conducted on 40 patients with prostate cancer who underwent external beam irradiation for which CT (Brilliance CT Big Bore, Philips, USA) and MRI (3T Ingenia Omega HP 5.1.7.3/5.3.1.1, Philips, Netherlands) were acquired on the same day in the treatment position. To generate sCTs, a 3D Cartesian multi-echo spoiled gradient echo (SPGR, Fig1) was acquired with the imaging parameters reported in Tab1.
Complex Cartesian data were retrospectively undersampled to simulate 3D GA radial stack-of-stars trajectories by applying the adjoint of the non-uniform fast Fourier transform (NUFFT)6: a fully sampled GA radial acquisition was simulated on the reconstructed data using 703 spokes, meaning an acquisition time of 358 s without considering parallel imaging accelerations. Also, we simulated undersampled GA acquisition applying the NUFFT followed by a Ram-Lak filter for density compensation for 12 undersampling factors (R) by considering the first acquired spokes (Fig2).
A conditional GAN (cGAN)7 was trained to translate the magnitude of the first echo to CT for each R (obtaining a total of 13 trained models considering the fully sampled case). Before training, CTs were rigidly registered to MRI, and the MR images were normalised. The network was trained on 20 randomly selected prostate patients in 2D transverse slices. To test the performance of the network, all the trained models were applied to the remaining patients (20=test set). Image evaluation was performed on the sCTs calculating the mean absolute error (MAE) and mean error (ME) of sCT against CT in the joint body contours of CT and sCT. To investigate the geometrical accuracy of sCTs, dice indices of the body contours and bones (sCT>200HU) were calculated. Patients with hip implants (2) were excluded from this analysis.
Equivalence test (difference<1σ) on the MAE of the sCT generated with R=1 was performed to identify the highest undersampling factors leading to accurate sCT generation. Dose recalculations of clinical 5-beam 10MV intensity-modulated RT prostate plan with a prescribed dose of 35x2.0Gy to the target were performed in Monaco (v5.11.02, Elekta AB, Sweden) for five patients in the test set on the CT and sCT obtained from training with R=1 and high undersampling factor. Dose distributions were subsequently analysed through voxel-based dose differences.
Results & Discussion
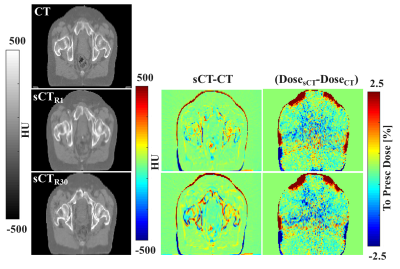
Training for 200 epochs required about 13.5 hrs on a GPU Tesla P100 (NVIDIA, USA) and single-patient sCT generation required <7s. An example of sCT generated from all the undersampling factors is provided in Fig2, where mode collapse8 can be observed for R≥80.
Image Evaluation: Average MAE was 44±7HU (±1σ, range: 35;61HU) for the case of a fully sampled radial acquisition. The errorbar in Fig3 shows the increase of MAE as a function of the undersampling factor. Up to R=20, the MAE was within 1σ with respect to the fully sampled case. We expect that R in the range 20-30 may be used without obtaining significant differences in the image evaluation and dose comparison.
Dose Evaluation: Average dose difference (CT-sCT) was 0.0±0.5% (range: -0.7;0.6%) and 0.1±0.6% (range: -0.7;0.7%) in the high dose region (dose >90% of the prescribed dose) for R=1 and 30, respectively, which is in line with dose differences generally accepted for MR-based radiotherapy planning1. Dose differences were within 0.5% between the two considered undersampling factors (Fig4).
Conclusions
Accurate MR-based dose calculation using a 2D cGAN for sCT generation is feasible for prostate cancer patients up to R=30. Undersampled GA radial acquisitions combined with a GAN-based sCT generation lead to short acquisition times (~12s = acquisition time reduction ~97%) and sCT generation times ~7s for the sequence used in this work. Prospectively data will be acquired to further validate these results. Concluding, we demonstrated that a cGAN can accurately generate sCT for undersampled GA radial acquisitions even in the presence of large aliasing artefacts.Acknowledgements
Jan J W Lagendijk provided general support to the research.References
[1] Edmund JM & Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol 2017;12(1):28.
[2] Maspero M, et al. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys Med Biol 2018; 63(18):185001-13.
[3] Feng L et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014;72(3):707-17.
[4] Winkelmann S, et al. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26(1):68-76.
[5] Hollingsworth KG. Reducing acquisition time in clinical MRI by data undersampling and compressed sensing reconstruction 2015 Phys Med Biol;60(21):R297-322.
[6] Fessler JA, Sutton BP. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Transactions on Signal Processing. 2003 Feb;51(2):560-74.
[7] Isola P et al. Image-to-Image Translation with Conditional Adversarial Networks, 2016 arXiv:1611.07004.
[8] Thanh-Tung H et al. On catastrophic forgetting and mode collapse in Generative Adversarial Networks. 2018 arXiv:1807.04015v2.
Figures