2151
Acceleration Factor Analysis of Different Dedicated 8Tx/16Rx Coil Arrays for Cardiac MRI at 7TMaria Roxana Stefanescu1, Maxim Terekhov1, Ibrahim A. Elabyad1, David Lohr1, Michael Hock1, and Laura Maria Schreiber1
1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
Signal-to-noise ratio (SNR) in cardiac MRI (cMRI) is a parameter associated with the field strength, the parallel imaging (PI) acceleration factor and the geometry of receive array element arrangement (g-factor). The purpose of this work was to evaluate four different arrays with symmetrical and asymmetrical distribution of 16RX and 8TX elements ex-vivo in swine at 7T MRI. The asymmetrical design proved to be superior with regard to SNR and image homogeneity.
Introduction
Possibilities to increase the MRI signal rely, among other factors, on higher field strength, optimization of receive properties of the RF-coil, and on the geometry of receive array element positioning of the receive coil (g-factor) [1]. The first prerequisite can be fulfilled by using 7T, although the challenges at that field strength are not always rewarded. Moreover, cardiac heart motion and the surrounding tissue such as the lung affect the image quality. The standard cMRI pulse sequences actively use integrated parallel receive technique (iPAT) which improves the acquisition speed, leading to an SNR penalty proportional to the square root of the acceleration factor and g-factor of the array. Therefore, the characterization of the noise amplification is an important aspect of testing the novel designs of the receive coil arrays for cMRI. Performing the measurements in an ex-vivo swine allows consistent conditions using high-resolution and varying acceleration factors, while avoiding cardiac and respiratory motion. Large animal experiments enable long measurement times and parameters settings not feasible in human. The receive properties of four newly designed TX/RX arrays were evaluated by comparing SNR values. This allows to step toward to the optimal coil geometry at 7T in swine.Methods
All measurements were performed on a 7T MAGNETOM™ Siemens Terra whole-body scanner equipped with a 16-kW radiofrequency amplifier for 8-channel parallel transmit (pTx) mode. The four novel coil arrays [2-4] were designed to have a curved housing to fit the body shape of the swine thorax. The MR measurements were performed using 4-different 8Tx/16Rx RF coil arrays. The first three coil designs (Design 1 , 2 & 3) have two independent anterior and posterior arrays, each array is composed of 8-elements. Design 1, which is used as a reference for comparison, is characterized by symmetrical rectangular elements for both top and bottom arrays. Design 2, an asymmetric coil design with reversed and mirrored elements, allows good decoupling and B1+-field localizations. Design 3 is composed of 8-elements having a circular shape coil. The fourth design consist of only one part having one half-elliptical shape housing (Xrad/Yrad=17/21-cm), 7 of the distributed 16-elements elements are reversed and mirrored around the central two-elements. All arrays demonstrate the feasibility of performing cMRI ex-vivo in swine of about 46kg. The swine was provided for ex-vivo MRI measurements after it was euthanized according to the project approval 55.2DMS2532-2-664 (Government of Lower Franconia, Germany). The analysis of SNR was carried out on CINE images including short axis (SA) and long axis (LA). Cardiovascular GRE pulse sequence measured with a standard turbo-FLASH pulse sequence (TE/TR=4.7/69.52ms for iPAT=2; TE/TR=4.7/78.21ms for iPAT=3; TE/TR=4.7/78.75ms for iPAT=4; FOV=320mm; FA=45°; resolution=0.3x0.3x6mm; 8 averages; 1 slice; BW=296Hz/Px). Parallel imaging methodology used for accelerating MR image acquisitions was Generalized Auto-Calibrating Partially Parallel Acquisition (GRAPPA) [5]. Six regions-of-interest (ROI) were selected for each direction and each iPAT-factor from which the averaged signal was divided by the averaged noise and standard deviation (STD) signal divided by STD noise.Results
Cardiac CINE images (SA and LA) acquired using Design 1 (Figure 1e, 1E) show a high variation of SNR due to the imaging plane and the position of the ROI. Design 1 with traditional rectilinear elements shows both strong variation of signal across FOV and a poor g-factor leading to GRAPPA-induced artefacts at high accelerations. Figure 2 illustrates a high anterior-posterior gradient of SNR in both image orientations as measured with Design 2. The images of the symmetrical (“star” arrangement of elements) coil Design 2 suffer from the relatively strong signal intensity variation. Moderate differences between data at iPAT=2-3 on one hand, and iPAT=4 on the other hand, were observed. For the asymmetric array, Design 3 shows only small variation between SNR of ROIs positioned within the heart (Figure 3). In contrast to previously shown designs 1 and 2 the difference between low (2,3) and high (4) iPAT factors are relatively small. Design 4 (Figure 4) with largest dimensions and without bottom part, makes evident the minimal intensity variation over the FOV. In comparison to the smaller designs, it reveals practically negligible visible noise amplification over iPAT=2-4.Discussions & Conclusions
The obtained results demonstrate the superiority of the tightly arranged asymmetrical designs (Design 3 & 4) over the symmetric coil designs (Design 1 & 2). The asymmetric distribution of the elements mirrored and reversed around the center enable good decoupling among the nearest elements and thus provide optimal B1+-field homogeneity and high SNR. Design 4 has a superior g-factor in the region of the heart because all 16-elements were distributed on the same housing, thus minimizing parallel imaging artefacts and SNR penalty.Acknowledgements
This work was financial supported by the German Ministry of Education and Research (BMBF grants: 01EO1004, 01EO1504). We would like to thank Dr. Steffen Baltes for providing us with the ex-vivo swine.References
- Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR Imaging. J Magn Reson Imaging. 2012 Jul; 36(1): 55–72.
- Elabyad IA, Terekhov M, Stefanescu MR, Lohr D, Fischer M, Schreiber LM. Design and development of dedicated multichannel transceiver coil arrays for swine cardiac MRI at 7T: Initial ex-vivo results. ISMRM2019
- Elabyad IA, Terekhov M, Stefanescu MR, Lohr D, Fischer M, Schreiber LM. Design and evaluation of a novel 8Tx/16Rx symmetric coil array for cardiac MRI in large animals (pigs) at 7T: Investigation of decoupling using a common central ring. ISMRM2019
- Elabyad IA, Terekhov M, Stefanescu MR, Lohr D, Fischer M, Schreiber LM. A novel asymmetric 16-element pTx transceiver coil array: towards denser elements for improved RF-shimming and g-factor for parallel cardiac MRI in pigs at 7T. ISMRM2019
- Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002; 47:1202–1210.
Figures

Figure 1. Arrangement of the Tx-elements in the array
design is displayed in the left bottom side. short-axis (SA) (a-d) and long-axis
(LA) (A-D) planes: (a,A)-iPAT2, (b,B)-iPAT3, (c,C)–iPAT4 and (d,D)–iPAT4 including
the 6 blue region-of-interests (ROIs) from where was subtracted the mean signal
divided by the mean of the noise (red circle) used as a reference. (e,E)- SNR
plot for SA and LA, respectively, including iPAT2-4 and standard deviation for
each iPAT factor. ROI5 (Figure 1E) due to proximity to the side coil elements
displays as an outlier with increased signal for all iPAT factors.

Figure 2. The array design (Design2) is displayed
in the left bottom side. short-axis (SA) (a-d) and long-axis (LA) (A-D) planes:
(a,A)-iPAT2, (b,B)-iPAT3, (c,C)–iPAT4 and (d,D)–iPAT4 including the 6 blue
region-of-interests (ROIs) from where was subtracted the mean signal divided by
the mean of the noise (red circle) used as a reference. (e,E)- SNR plot for SA
and LA, respectively, including iPAT2-4 and standard deviation for each iPAT
factor.

Figure 3. The elements distribution of the array
(Design3) is displayed in the left bottom side. short-axis (SA) (a-d) and long-axis
(LA) (A-D) planes: (a,A)-iPAT2, (b,B)-iPAT3, (c,C)–iPAT4 and (d,D)–iPAT4 including
the 6 blue region-of-interests (ROIs) from where was subtracted the mean signal
divided by the mean of the noise (red circle) used as a reference. (e,E)- SNR
plot for SA and LA, respectively, including iPAT2-4 and standard deviation for
each iPAT factor.
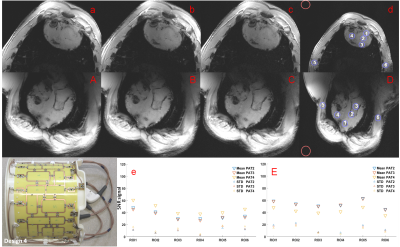
Figure 4. The array design (Design 4) is
displayed in the left bottom side. Short-Axis (SA) (a-d) and Long-Axis (LA)
(A-D) planes: (a,A)-iPAT2, (b,B)-iPAT3, (c,C)–iPAT4 and (d,D)–iPAT4 including
the 6 blue region-of-interests (ROIs) from where was subtracted the mean signal
divided by the mean of the noise (red circle) used as a reference. (e,E)- SNR
plot for SA and LA, respectively, including iPAT2-4 and standard deviation for
each iPAT factor.