2101
Fetal cardiac MRI using rapid gradient-spoiled spiral imaging at 1.5 and 3T1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 3NHLBI, National Institutes of Health, Bethesda, MD, United States, 4Heart Centre, The Hospital for Sick Children, Toronto, ON, Canada, 5Paediatrics, University of Toronto, Toronto, ON, Canada, 6Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Recent advances in image acquisition and reconstruction techniques have allowed for dynamic imaging of the fetal heart using balanced SSFP (bSSFP). Here we demonstrate the use of a center-out spiral trajectory that is gradient spoiled to maintain temporal resolution and decrease banding from bSSFP. This technique is applied in adult volunteers for quantification of contrast and image sharpness, and is further demonstrated in two fetal subjects to display real-time and CINE recons of the fetal heart.
Introduction:
CINE imaging of the fetal heart is constrained by the size of fetal cardiac anatomy, fast fetal heart rate, and various sources of motion including maternal respiration and gross fetal movement. Recent advances have enabled high resolution imaging of the fetal heart by employing undersampled acquisitions and reconstructions that compensate for motion1-5. In particular, radial balanced SSFP (bSSFP) acquisitions have been used in several studies to reconstruct real-time images1-4. However, bSSFP is sensitive to artifacts due to off-resonance, leading to telltale banding artifacts that are accentuated by blood flow and exacerbated at higher field strengths. These artifacts inhibit the use of 3T systems with their potential SNR benefits. The addition of gradient spoiling (i.e. GRE imaging) eliminates these artifacts but at the cost of reduced myocardial to blood pool contrast and increased TR, thus decreasing temporal resolution for real-time reconstructions. In this work, we investigate the use of a center-out spiral GRE sequence as an alternative to radial bSSFP for real-time and CINE fetal cardiac imaging. Spiral GRE imaging has many attractive qualities: gradient spoiling to reduce off-resonance effects, short TE with small gradient first moment (good for reducing flow-induced artifacts and maintaining SNR6), short TR to support high temporal resolution real-time imaging, and oversampling of the k-space center to reduce sensitivity to fetal motion. We present qualitative and quantitative comparisons of real-time and CINE images from radial bSSFP, radial GRE and spiral GRE sequences acquired in healthy adults. The feasibility of fetal cardiac spiral GRE at 1.5T and 3T is then demonstrated.Methods:
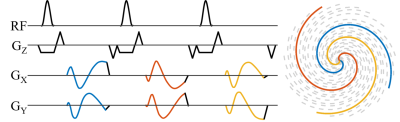
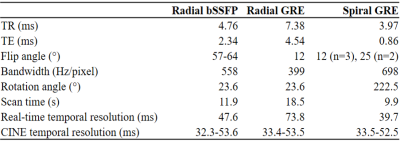
Acquisition: A standard Cartesian GRE gradient-spoiled sequence was modified to acquire rapid center-out variable density spiral readouts7. Spiral readouts were continuously rotated by 2 times the golden angle8 (Figure 1). Following informed consent, five healthy adult volunteers (1F, 29±3 years) were scanned on a 3T clinical system (MAGNETOM PrismaFIT, Siemens Healthcare, Erlangen, Germany) under breath-hold conditions in one mid short axis and one four chamber slice using three acquisition strategies: our standard radial bSSFP, radial GRE, and the spiral GRE sequence (Table 1). Each acquisition consisted of 2500 readouts at FOV = 256x256x5 mm3 and spatial resolution = 1x1x5 mm3. Due to the lack of a prewinder, TR was shortest in the center-out spiral GRE sequence.
Finally, as a proof of concept, spiral GRE was performed in a single short axis slice in two fetal subjects at 1x1x5 mm3: one at 1.5T (AvantoFIT, Siemens), TR/TE (ms) = 7.33/1.74, α = 15°, FOV = 320x320x5 mm3) and one at 3T (PrismaFIT, Siemens: TR/TE (ms) = 3.55 /0.86, α = 25°, FOV = 256x256x5 mm3). Both subjects were at 40 weeks gestational age and were previously diagnosed with congenital heart disease by echocardiography.
Reconstruction: All reconstructions were performed offline in MATLAB (MathWorks, Natick, MA, USA). Errors in spiral trajectories were corrected using the pre-measured system gradient impulse response function9. Compressed sensing was used for both real-time and reordered CINE reconstructions using spatial total-variation, temporal total-variation, and spatial wavelet regularizers.
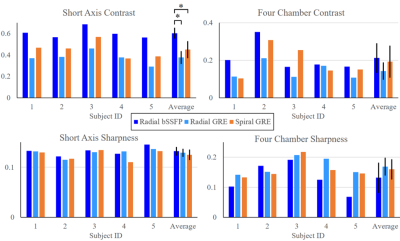
Technique comparison: In CINE recons for all adult scans, image quality was compared using metrics for contrast and sharpness. Myocardial – blood pool contrast was measured from manually drawn ROIs, and sharpness was evaluated as previously described10. Statistical comparisons between each technique and for each metric were performed using one-way ANOVA (with p<0.05 considered statistically significant).
Results:
Figure 2 demonstrates real-time breath-held short-axis images across techniques in one adult volunteer. Corresponding full CINE recons from images are also shown in Figure 2. Blood-myocardial contrast results are displayed in Figure 3. In short axis slices, image sharpness was similar across presented techniques, yet radial bSSFP blood-myocardial contrast outperforms radial GRE and spiral GRE. In four chamber slices, no contrast differences were observed across techniques. No statistically significant differences were found in blood-myocardial sharpness across techniques. Figure 4 displays animated results from spiral GRE images of the fetal heart acquired at 1.5T and 3.0T. Dynamic cardiac motion is well depicted by both examples demonstrating the feasibility of spiral GRE for fetal cardiac MRI.
Discussion and conclusion:
Golden angle center-out spiral GRE is a potentially useful alternative to radial sequences for improving temporal resolution (compared with radial GRE) and mitigating off-resonance effects (compared with radial bSSFP) in cardiac imaging. Flip angle for spiral GRE was chosen as a compromise between typical values for GRE (12°) and bSSFP (~60°). Future optimization of flip angle will be explored to improve the results presented here. Preliminary results applying spiral GRE to the fetal heart yielded dynamic cardiac motion with visually sharp structures demonstrating the promise of this method for improving fetal cardiac imaging at both 1.5 and 3.0T.Acknowledgements
No acknowledgement found.References
1. Roy CW, Seed M, Macgowan CK. Accelerated MRI of the fetal heart using compressed sensing and metric optimized gating. Magn Reson Med 77, 2125-2135 (2017).
2. Chaptinel J, et al. Fetal cardiac cine magnetic resonance imaging in utero. Sci Rep 7, 15540 (2017).
3. Haris K, et al. Self-gated fetal cardiac MRI with tiny golden angle iGRASP: A feasibility study. J Magn Reson Imaging 46, 207-217 (2017).
4. Kording F, et al. Dynamic fetal cardiovascular magnetic resonance imaging using Doppler ultrasound gating. J Cardiovasc Magn Reson 20, 17 (2018).
5. van Amerom JFP, et al. Fetal cardiac cine imaging using highly accelerated dynamic MRI with retrospective motion correction and outlier rejection. Magn Reson Med 79, 327-338 (2018).
6. Hoerr V, Nagelmann N, Nauerth A, Kuhlmann MT, Stypmann J, Faber C. Cardiac-respiratory self-gated cine ultra-short echo time (UTE) cardiovascular magnetic resonance for assessment of functional cardiac parameters at high magnetic fields. J Cardiovasc Magn Reson 15, 59 (2013).
7. Lee JH, Hargreaves BA, Hu BS, Nishimura DG. Fast 3D imaging using variable-density spiral trajectories with applications to limb perfusion. Magn Reson Med 50, 1276-1285 (2003).
8. Kowalik G, Courot A, Steeden JA, V. M. Golden-Angle Spiral Sparse Parallel Phase-Contrast MR acquisition with an on-line fast GPU based reconstruction for high resolution real-time cardiovascular assessments. In: International Society for Magnetic Resonance in Medicine, Honolulu, HI, USA (2017).
9. Campbell-Washburn AE, Xue H, Lederman RJ, Faranesh AZ, Hansen MS. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn Reson Med 75, 2278-2285 (2016).
10. Ahmad R, Ding Y, Simonetti OP. Edge Sharpness Assessment by Parametric Modeling: Application to Magnetic Resonance Imaging. Concepts Magn Reson Part A Bridg Educ Res 44, 138-149 (2015).
Figures