1972
Experimental Validation of 4D Flow MRI for the Assessment of Flow Dynamics within a Patient-Specific Intracranial Aneurysm Model using Tomographic Particle Image Velocimetry1Mechanical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
4D flow MRI has shown to be a feasible tool for assessing hemodynamics in different vascular territories with high spatial resolution. This study aimed to compare velocity components and magnitudes within a patient-specific intracranial aneurysm in-vitro model using 4D Flow MRI and a ground truth experimental technique, tomographic particle image velocimetry (tomo-PIV). Tomo-PIV offers higher temporal and spatial resolution allowing the assessment of intra-cycle differences caused by pulsatile flow. This analysis was done to assess the ability of 4D Flow MRI to capture the complex flows within an intracranial aneurysm considering the cardiac cycle averaging of the data.
Introduction
Intracranial aneurysms are a type of cerebrovascular disease where a weakening in the arterial wall leads to a local ballooning of the vasculature. Since aneurysm rupture has been suggested to be related to aneurysm geometry, morphology1,2, and high flow activity3, understanding the aneurysm-specific hemodynamics is necessary in a diagnostic tool. 4D Flow MRI can non-invasively capture three-directional velocity throughout the cardiac cycle, allowing for accurate blood velocity and flow rate measurements, even in complex flow regimes.4,5 Recent studies have used tomographic particle image velocimetry (tomo-PIV)6 to study physiological and pathological flows in vitro by using cameras to capture the movement of particles illuminated by a laser to optically measure velocities in 3D.7,8,9 Tomo-PIV offers an instantaneous volumetric measurement of flow, making it relevant to studies with complex anatomical geometries, such as intracranial aneurysms. The purpose of this study is to further assess the ability of 4D Flow MRI to capture specific intracranial aneurysm flow patterns.Methods
Aneurysm Models: Patient-specific, 3D digital subtraction angiography images were obtained under an institutional approved IRB protocol. A data set of anterior circulation internal carotid aneurysm were retrospectively selected from a database of clinical exams. Projections were reconstructed into a 3D volume and segmented. The geometry was scaled by 2 and 3D printed, using a dissolvable material, after being post-processed to correct for surface imperfections. A lost-casting method was performed to create the silicone model. Figure 1 illustrates this process.
MR Imaging: The in vitro model was perfused using a positive displacement pulsatile pump (Figure 2A) in line with a hemodynamic conditioning head (BDC Laboratories), and scanned on a clinical 3T scanner (Discovery MR 750, GE Healthcare) (Figure 2B), using an 8-channel high resolution head coil. 4D flow MRI was performed with a 5-pt radial-undersampled technique, PC-VIPR.10 Imaging parameters were as follows: imaging volume: 24 x 24 x 24 cm; 0.625 mm isotropic spatial resolution; TR/TE = 7.5/3.2 ms; VENC = 50 cm/s; scan time 7 minutes. MRI was performed while a solution of water and glycerol circulated through the model at 0.7 L/min simulating a heart rate of 60 beats per minute.
Tomographic PIV: The PIV system (LaVision) consisted of a laser beam projected in the direction perpendicular to three high-speed cameras (Figure 2C-D). Flow inlet conditions were replicated to those used for the MRI experiment. A total of 9 cardiac cycles were acquired on three different days to control for acquisition variability and obtain intra-cycle differences. The resulting spatial resolution was 0.2 x 0.2 x 0.2 mm.
Data Analysis: Data from 4D Flow MRI and tomo-PIV were visualized and quantified in Ensight (Anysys Inc.). Velocity fields within the aneurysm sac were qualitatively and quantitatively compared for time-resolved and time-averaged data reconstructions. For qualitative comparison, 9 cardiac cycles from tomo-PIV were averaged into one data set to resemble time-resolved 4D Flow MRI, which averages several cardiac cycles into one. A pixel-by-pixel velocity comparison was done with a two tailed t-test to assess any cycle-by-cycle variations in flows between the 9 cardiac cycle. For quantitative comparison, the average velocity along the data set was compared to the time-averaged obtained from 4D Flow MRI.
Results
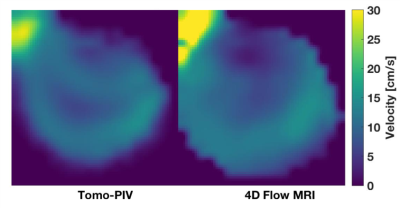
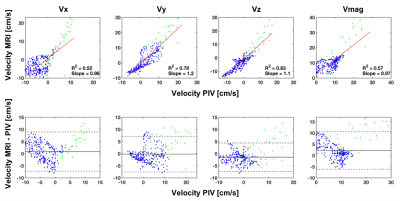
Quantitative and qualitative agreement was observed between 4D flow MRI and tomo-PIV alongthe cardiac cycle (Figure 3). Significant intra-cycle differences were found (p-value < 0.05) between velocity magnitudes measured with tomo-PIV at peak systole and end diastole in 78% of the pair-wise comparisons. A pixel-wise comparison at the same plane location was made for time-averaged data (Figure 4). Linear regression and Bland-Altman plots (Figure 5) illustrate the relationships for each velocity component and magnitude.Discussion
This study showed the feasibility of creating patient-specific in vitro experiments of intracranial aneurysms to assess 4D Flow MRI. Although intra-cycle differences were obtained between the 9 cardiac cycles, these were not noticeable qualitatively. The selected VENC showed to be ideal to analyze flow within the aneurysm sac, however a higher VENC would have been necessary to avoid aliasing at other regions. This suggest future studies with acquisition protocols with multi VENC.Conclusion
4D Flow MRI is a valuable medical imaging technique that has shown promising results assessing in vivo hemodynamics. PIV provides great insight into the velocity field within the model, using a superior acquisition rate and spatial resolution. The use of 4D Flow MRI has potential to be a helpful, non-invasive tool to study intracranial aneurysm flow and with future work, possibly other physiological parameters that may induce rupture.Acknowledgements
We acknowledge support from the K12 (K12DK100022), UW Radiology R&D and GE Healthcare.References
1. Kang H, Ji W, Qian Z, et al. Aneurysm characteristics associated with the rupture risk of intracranial aneurysms: A self-controlled study. PLoS One., 2015.
2. Qiu T, Jin G, Xing H, Lu H. Association between hemodynamics, morphology, and rupture risk of intracranial aneurysms: a computational fluid modeling study. Neurol Sci., 2017.
3. Cebral J, Ollikainen E, Chung BJ, et al. Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. Am J Neuroradiol, 2017.
4. Markl, M., A. Harloff, T. A. Bley, M. Zaitsev, B. Jung, E. Weigang, M. Langer, J. Hennig, and A. Frydrychowicz. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J. Magn. Reson. Imaging 25:824–31, 2007.
5. Rebergen, S. A., E. E. Van Der Wall, and J. Doornbos. Magnetic resonance measurement of velocity and flow: Technique, validation, and cardiovascular applications. 1993.
6. Elsinga, G. E., F. Scarano, B. Wieneke, and B. W. Van Oudheusden. Tomographic particle image velocimetry. Exp. Fluids 41:933–947, 2006.
7. Hasler, D., A. Landolt, and D. Obrist. Tomographic PIV behind a prosthetic heart valve. Exp. Fluids 57:1–13, 2016.
8. Buchmann, N. A., C. Atkinson, M. C. Jeremy, and J. Soria. Tomographic particle image velocimetry investigation of the flow in a modeled human carotid artery bifurcation. Exp. Fluids 50:1131–1151, 2011.
9. Medero, R., C. Hoffman, and A. Roldán-Alzate. Comparison of 4D Flow MRI and Particle Image Velocimetry Using an In Vitro Carotid Bifurcation Model. Ann. Biomed. Eng., 2018.
10. Johnson, K.M., Markl, M. Improved SNR in phase contrast velocimetry with five-point balanced flow encoding. Magn. Reson. Med.; 63, 349–355, 2010.
Figures