1857
Breast DCE-MRI using Radial Acquisition with Data-Driven Model Consistency Condition Reconstruction1Department of Medical Physics, University of Wisconsin Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin Madison, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin Madison, Madison, WI, United States, 4Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 5MR Engineering, GE Healthcare, Waukesha, WI, United States
Synopsis
Advanced data acquisition and reconstruction methods have been proposed to improve temporal and spatial resolution DCE imaging for breast. However, few studies have validated these techniques. In this work we propose a radial acquisition with MOCCO reconstruction and assess performance of these methods using a simulated digital reference object breast phantom including pharmacokinetic simulation. An improvement to the MOCCO formulation is proposed using independent component analysis to learn the temporal components. Finally, the extended Tofts model was used to extract pharmacokinetic parameters from time series with different temporal resolution. Dynamic data were then use for pharmacokinetic (PK) fitting to assess the ability to recover PK parameters.
Introduction
Dynamic contrast-enhanced (DCE) MRI provides the greatest sensitivity of any modality for the detection of breast cancer1-3 however its insufficiently high specificity can result in indeterminate findings requiring biopsy and/or imaging follow-up4-5. Quantitative pharmacokinetic (PK) modeling of DCE-MRI has shown promise for differentiating benign and malignant lesions2. However, high temporal resolution is required for accurate estimation of the PK parameters while high spatial resolution is needed to detect and characterize lesions. Studies have shown the potential of achieving high spatial-temporal resolution breast images by using stack-of-stars radial acquisition with advanced reconstruction methods. Recently, a data-driven low-rank-based method exploiting spatial-temporal correlations was used to generate high temporal resolution images (10 seconds/frame)6. However, the temporal components for contrast kinetics learned from principle component analysis (PCA) suffered from strong oscillations due to periodic cardiac motion. Here, we propose a novel approach for learning temporal components through complex independent component analysis (ICA)7 to improve reconstruction/quantification performance in the presence of cardiac motion.Theory
Time-resolved images were reconstructed using a modified MOCCO algorithm8. The underlying temporal model was obtained from the fully-sampled central k-space data using progressive learning with cubic spline approximation9-10 followed by either PCA (MOCCO+PCA) or ICA (MOCCO+ICA); the latter to allow for separation of temporal behaviors describing contrast propagation from cardiac motion. Unlike PCA, ICA does not rank components, therefore an automated selection was performed based on total variation of temporal components, resulting in models of order 2 to 4Methods
Simulations: A time-resolved acquisition was simulated by using a digital reference object (DRO) breast phantom11 with a 448x448x142 matrix that included cardiac motion (Fig. 1). The simulated radial data with golden angle ordering were reconstructed at 10-s and 5-s temporal resolution, corresponding to 20 and 10 projections/frame. For comparison, SENSE reconstruction was performed at 40-s temporal resolution and for idealized reference fully sampled simulating single slice acquisition at 5-s temporal resolution. Results were evaluated using normalized root-mean-square error (nRMSE) and Tenengrad measure of sharpness12.
Quantitative analysis: The signal from regions-of-interest (ROIs) in each lesion were converted to gadolinium (Gd) concentration, based on an assumed breast tissue T1=1266ms13. The extended Tofts model was used to fit The Gd concentration-time curves were fitted to the extended Tofts model14 using in-house implementation of Levenberg-Marquaradt algorithm. A population-averaged arterial input function (AIF)15 was used for PK analysis. Volunteers: Two patient volunteers were imaged during contrast injection (gadobenate dimeglumine, Multihance; Bracco Inc, Milan, Italy) on a clinical 3T MRI (Signa Premier, GE Healthcare, Waukesha, WI) using a 16-channel breast coil (Sentinelle, Invivo International, Gainsville, FL) for this IRB-approved, HIPPA- compliant study. A 3D radial stack-of-stars gradient echo sequence (TR/TE= 5.5/2.4 ms, FOV= 340 x 340 mm, 448x448 in-plane matrix, 142 z-phase encodes, 1.5x out-of-plane acceleration) was used to collect 896 unique radial projections with golden-angle ordering. The data were reconstruction with MOCCO+ICA at 5-s temporal resolution.
Results
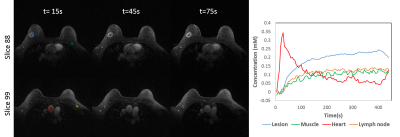
Simulations: Similar image quality was observed for all undersampled reconstructions (Fig. 2) with MOCCO+ICA producing lowest nRMSE values in the breasts and axilla. Residual low-intensity streaking artifacts visible near the heart at 80s and 140s is due to the peak contrast in the heart chamber and data inconsistency due to cardiac wall motion. The use of temporal components free from oscillatory cardiac motion for MOCCO+ICA resulted in 7% improvement in Tenengrad measure of sharpness over MOCCO+PCA images. Importantly, MOCCO+ICA resulted in temporally regular Gd tissue concentration curves (Fig. 3), while oscillating curves from MOCCO+PCA significantly underestimate signal intensity. For the quantitative analysis (Fig. 4), 5-s and 10-s temporal resolution series showed better prediction of Ktrans than 40-s temporal resolution. However, there were larger differences in Ve when compared to reference images and Vp showed overall larger variation for all three time series. Volunteers: Images from patient volunteer 1 allow visualization of an enhancing invasive ductal carcinoma without evidence of significant undersampling artifacts (Fig. 5). Individual ROIs placed on specific tissues of interest demonstrate the ability to capture the unique temporal dynamics.Discussion and conclusions
In this study, we proposed a novel way to combine radial acquisition with low-rank MOCCO reconstruction with ICA-based temporal model to obtain high spatial-temporal resolution breast images. Simulations and in vivo data for effective temporal resolutions down to 5-s, equivalent to 88x angular undersampling, were shown. MOCCO+ICA produced similar image quality as SENSE and MOCCO+PCA, while reducing temporal blurring/oscillations and maintaining temporal fidelity in the ROI analysis of the contrast enhancement in the simulation. PK modeling showed less deviation from reference Ktrans values for the 10-s and 5-s temporal resolution MOCCO+ICA data compared to results from the conventional 40-s temporal resolution.Acknowledgements
The authors wish to acknowledge GE Healthcare and the RSNA Research and Education Foundation for their research support and the following grant support NIH-R21EB018483 and NIH-R01EB027087References
- Kuhl, C. K., Schrading, S., Strobel, K., Schild, H. H., Hilgers, R. D., & Bieling, H. B. (2014). Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. Journal of Clinical Oncology, 32(22), 2304-2310.
- Partridge, S. C., Stone, K. M., Strigel, R. M., DeMartini, W. B., Peacock, S., & Lehman, C. D. (2014). Breast DCE-MRI: influence of postcontrast timing on automated lesion kinetics assessments and discrimination of benign and malignant lesions. Academic radiology, 21(9), 1195-1203.
- Kim, E. J., Kim, S. H., Kang, B. J., Choi, B. G., Song, B. J., & Choi, J. J. (2014). Diagnostic value of breast MRI for predicting metastatic axillary lymph nodes in breast cancer patients: diffusion-weighted MRI and conventional MRI. Magnetic resonance imaging, 32(10), 1230-1236.
- Huang, W., Fisher, P. R., Dulaimy, K., Tudorica, L. A., O’Hea, B., & Button, T. M. (2004). Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology, 232(2), 585-591.
- Warren, R. M., Pointon, L., Thompson, D., Hoff, R., Gilbert, F. J., Padhani, A., ... & Leach, M. O. (2005). Reading protocol for dynamic contrast-enhanced MR images of the breast: sensitivity and specificity analysis. Radiology, 236(3), 779-788.
- Wang P, Strigel R, Velikina J, Samsonov A, Bancroft L, Wang K, Cashen T, Johnson K & Holmes J. Feasibility of High Spatial and Temporal Resolution Breast DCE-MRI using Radial Acquisition with Data-Driven Model Consistency Condition Reconstruction. Proceedings of the 26th ISMRM Scientific Meeting 2018. Paris France, 2018.
- Novey, M., & Adali, T. (2008). On extending the complex FastICA algorithm to noncircular sources. IEEE Trans. Signal Processing, 56(5), 2148-2154.
- Velikina, J. V., & Samsonov, A. A. (2015). Reconstruction of dynamic image series from undersampled MRI data using data‐driven model consistency condition (MOCCO). Magnetic resonance in medicine, 74(5), 1279-1290.
- Velikina J, Alexander A, Salmons J, Raimy E, Purnell T, Kecskemeti S, & Samsonov A. Ultrafast Speech Imaging at High Spatial Resolution using Model-Consistency Condition Reconstruction with Progressive Temporal Basis Learning. Proceedings of the 26th ISMRM Scientific Meeting 2018. Paris France, 2018.
- De Boor, C., De Boor, C., Mathématicien, E. U., De Boor, C., & De Boor, C. (1978). A practical guide to splines (Vol. 27, p. 325). New York: Springer-Verlag.
- Henze LC, Moran CJ, Smith MR, Kelcz F, Samsonov A, Fain SB, Block WF. Digital Breast Phantom for Evaluating Dynamic Accelerated Imaging Methods. Proceedings of the 18th ISMRM Scientific Meeting 2010. Stockholm Sweden, 2010.
- Krotkov E. Focusing. Int J Comput Vis. 1988;1(3):223–237.
- Rakow‐Penner, R., Daniel, B., Yu, H., Sawyer‐Glover, A., & Glover, G. H. (2006). Relaxation times of breast tissue at 1.5 T and 3T measured using IDEAL. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 23(1), 87-91.
- Tofts, P. S., Brix, G., Buckley, D. L., Evelhoch, J. L., Henderson, E., Knopp, M. V., ... & Port, R. E. (1999). Estimating kinetic parameters from dynamic contrast‐enhanced T1‐weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 10(3), 223-232.
- Barboriak, D. P., MacFall, J. R., Viglianti, B. L., & Dewhirst Dvm, M. W. (2008). Comparison of three physiologically‐based pharmacokinetic models for the prediction of contrast agent distribution measured by dynamic MR imaging. Journal of Magnetic Resonance Imaging, 27(6), 1388-1398.
Figures