1505
Design and Evaluation of a Novel 8Tx/16Rx Symmetric Coil Array for Cardiac MRI in Large Animals (Pigs) at 7T: Investigation of Decoupling Using a Common Central Ring1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
A dedicated 8Tx/16Rx coil array was designed and tested for cardiac magnetic resonance imaging (cMRI) ex-vivo pigs at 7T. The cardiac array is composed of 16-elements with physically independent anterior and posterior parts. The anterior array is composed of 8-elements and resembles a symmetric circular shape coil. The central four-elements are decoupled using a common central ring and shared decoupling capacitors. The posterior array was built using 4×2 rectangular symmetric elements configuration. Ex-vivo high-resolution cardiac images were acquired with 0.3 mm x 0.3 mm in plane resolution. The dedicated coil enhances the SNR within the heart by about four-times compared to a commercial human coil.
Introduction
Developing novel multichannel coil arrays for cMRI at 7T, each of these loop elements are selectively driven with its individual amplitude/phase to smooth the B1+ profile is an important option for RF-shimming 1-3. In order to effectively use RF-shimming, the coil array should have spatially separated B1+-field within the heart. One important aspect for efficient RF-shimming and parallel imaging is the decoupling of the composed elements. Nevertheless, several groups successfully performed human body imaging at UHF using different types of coil arrays (e.g., local multichannel loops 4-7 and dipole antenna arrays 8-10. Performing cMRI in swine plays an important role in the development of new MRI methodology towards preclinical and clinical imaging at 7T. However, pigs have a different thorax shape than humans, which makes the usage of the human coils in pigs cMRI is suboptimal solution due to less loading and non-optimized loop elements. Therefore, we propose in this work a dedicated 8Tx/16Rx coil array for cMRI in pigs at 7T for high SNR and optimal resolution to characterize in the future myocardium infarct.Materials and Methods
The novel coil design is composed of centrally symmetric octagonal four-elements decoupled using a common central ring. The ring is split to accommodate four decoupling capacitors (C2d) (Figure 1a) 11. Each element is octagonal in shape with 4×8.6 cm2. Each two neighboring elements are orthogonally oriented to have 90° geometrical angle and decoupled using the decoupling capacitors (C1d). The central four-elements are surrounded by another four symmetric triangular shape elements of 5×5 cm2. The central elements 1, 2, 3 and 4 were decoupled from the neighboring elements (5, 6, 7 and 8) using capacitive decoupling (C3d) in addition to a decoupling gap of 9mm. The external diameter for the circular shape coil array is 19.8 cm. The posterior array was built using 4×2 rectangular symmetric elements configuration. Larger coil elements with 5×10 cm2 were chosen for all of the four-element pairs (Figure 1b). Due to the increased size, we can exploit the larger penetration depth of larger coils and thereby effectively reach the center of the heart despite the larger distance to the object of interest. In total, the symmetric posterior array measured 21.2×26.8 cm2 (Figure 1b). EM-simulations were performed using CST-Microwave-Studio. The coil has copper track width of 4 mm etched on a 0.3 mm FR4-PCB. RF-circuit Co-simulation was used for coil design by modeling all feeding ports, tuning, matching, and decoupling capacitors by 50 Ω discrete face ports 12. The final total numbers of mesh cells is 35.3 million. The measurements were performed on a 7T whole-body MAGNETOM Siemens Terra scanner equipped with a 16-kW RF amplifier for 8-channel pTx mode. The novel coil array was designed to have a curved housing for both anterior and posterior sections to demonstrate the feasibility of performing cMRI for pigs (Figure 2).Results and Discussion
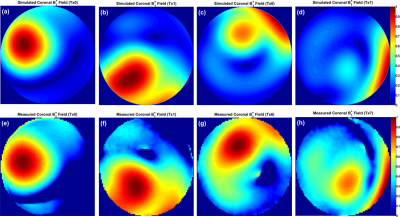
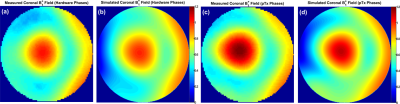
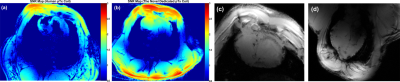
Due to the advantageous arrangement of elements in the novel coil design and by optimizing the decoupling capacitors (C1d and C2d), the reflection coefficients of unloaded and flat array (S11, S22, S33, and S44) are -25 dB and the transmission coefficients S21 and S31 are -23.5 dB (Figure 2c). The measured Qlo/Qun for elements 1 and 2 are 54/72, for the elements 3 and 4 are 54/65, for the elements 5, 6, 7 and 8 are 60/75. For the bottom array the Qlo/Qun ratio is 0.55. The B1+-field for each two individual elements was combined in one Tx-channel to form the Tx-pairing channels and was normalized to its own maximum value. Figure 3 displays the normalized coronal B1+-field distribution for all individual 4-channels of the top array. The pattern of the B1+-field is similar for all channels and matches very well even with the destructive interference locations. Figure 4 shows the simulated and measured combined B1+-field for all 8-channels. A good agreement between simulation and measurement was achieved. An improvement of about 15% was achieved in the B1+-field intensity with pTx-phases compared to the implemented phases. The mean SNR within the heart region from the novel design was 40 compared to 10 from a commercial human coil (Figure 5a, b). High-resolution images with in-plane pixel resolution of 0.3 mm x 0.3 mm were obtained using both coils (Figure 5c, d). The pTx-system can be used for additional improvement in the B1+-field homogeneity.Conclusion
A dedicated transceiver 8Tx/16Rx coil array has been designed and evaluated in phantom and ex-vivo large animals for cMRI at 7T. A higher intrinsic SNR, advantageous B1+-field homogeneity with distinct B1+-field localizations has been obtained from the novel coil design. Since the ex-vivo images displayed an enhanced contrast and SNR, it is expected that the coil provides high image quality in-vivo.Acknowledgements
Financial support: German Ministry of Education and Research (BMBF, grants: 01EO1004, 01E1O1504).
The animals were provided for ex-vivo measurements after they were euthanized following their approved use in project 55.2 DMS 2532-2-664 (Regierung Unterfranken). Euthanasia was performed with intravenous application of 150 mg/kg pentobarbital under isoflurane anesthesia with fentanyl analgesia.
References
- Ibrahim, T. S. (2006). Ultrahigh-field MRI whole-slice and localized RF field excitations using the same RF transmit array. IEEE transactions on medical imaging, 25(10), 1341-1347.
- Mao, W., Smith, M. B., and Collins, C. M. (2006). Exploring the limits of RF shimming for high-field MRI of the human head. Magnetic Resonance in Medicine, 56(4), 918-922.
- Xin, S. X., Huang, Q., Gao, Y., et al., (2013). Fetus MRI at 7 T: Shimming Strategy and SAR safety implications. IEEE Transactions on Microwave Theory and Techniques, 61, 2146-2152.
- Thalhammer, C., Renz, W., Winter, L., et al., (2012). Two-dimensional sixteen channel transmit/receive coil array for cardiac MRI at 7.0 T: design, evaluation, and application. Journal of Magnetic Resonance Imaging, 36(4), 847-857.
- Dieringer, M. A., Renz, W., Lindel, T., et al., (2011). Design and application of a four-channel transmit/receive surface coil for functional cardiac imaging at 7T. Journal of Magnetic Resonance Imaging, 33(3), 736-741.
- Gräßl, A., Winter, L., Thalhammer, C., et al., (2013). Design, evaluation and application of an eight channel transmit/receive coil array for cardiac MRI at 7.0 T. European journal of radiology, 82(5), 752-759.
- Graessl, A., Renz, W., Hezel, F., et al., (2014). Modular 32-channel transceiver coil array for cardiac MRI at 7.0 T. Magnetic resonance in medicine, 72(1), 276-290.
- Raaijmakers, A. J. E., Luijten, P. R., and van Den Berg, C. A. (2016). Dipole antennas for ultrahigh-field body imaging: a comparison with loop coils. NMR in Biomedicine, 29(9), 1122-1130.
- Oezerdem, C., Winter, L., Graessl, A., et al., (2016). 16-channel bow tie antenna transceiver array for cardiac MR at 7.0 tesla. Magnetic resonance in medicine, 75(6), 2553-2565.
- Ertürk, M. A., Wu, X., Eryaman, Y., Van de Moortele, P. F., et al., (2017). Toward imaging the body at 10.5 tesla. Magnetic resonance in medicine, 77(1), 434-443.
- Perrier, A. L., Grenier, D., Pouzin, A., et al., (2012). Design of a two-channel NMR coil using an impedance transformation approach. IEEE Sensors Journal, 12(6), 1801-1808.
- Kozlov, M., and Turner, R. (2009). Fast MRI coil analysis based on 3-D electromagnetic and RF circuit co-simulation. Journal of magnetic resonance, 200(1), 147-152.
Figures