1500
Development and Test of an Optimized 8TX/16RX Array for Ultra-High Resolution Ex-Vivo Myocardial Tissue Characterization with 7T MRI : Initial Experience and Quality Assessment.1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
The MRI measurements of the excised hearts providing stable “ground truth” high resolution images are important part of cardiac MRI at ultra-high field. We report the initial results of testing an in-house developed multiple element transceiver array (mTA) with parallel transmit support optimized for submillimeter spatial resolution ex-vivo heart tissue characterization MRI at 7T. The array testing included SNR, B1-shimming, g-factor, T2* and DTI mapping with high parallel imaging acceleration factors. The designed 8TX/16RX array demonstrated high efficiency of both TX and RX properties for (ultra)high ex-vivo myocardial tissue characterization imaging at 7T with essential superiority to a commercial 1TX/32RX coil.
Introduction
Since the last decade ultra-high field (UHF) MRI (B0>7T) becomes an emerging tool of assessment of the human brain structure and function with spatial resolution and signal-to-noise ratio (SNR) superior to clinical 1.5/3T scanners. However, for cardiac MRI (cMRI) UHF remains challenging due to stronger inhomogeneities of B0 and B1+-fields induced by thorax tissue heterogeneity. Therefore, ex-vivo measurements may be an important step in both providing a stable “ground truth” reference and translation to human imaging in UHF cMRI. To achieve highest SNR and spatial resolution, an optimal TX/RX characteristic of hardware is crucial. We report initial results of testing an in-house developed multiple element transceiver array (mTA) for ultra-high resolution ex-vivo heart tissue characterization MRI at 7T.Method
The developed mTA was designed with reversed/mirrored elements around the center. The decoupling was accomplished by common conductor and shared capacitors for some the central elements and by using gaps and capacitive decoupling for the surrounding elements [1]. The 16 elements of top and bottom parts are paired for 8 available TX-channels (Figure 1A) providing 8TX/16RX array configuration. The final dimensions of the array are shown on Figure 1B. The array parameters were simulated and optimized with CST software (CST, Darmstadt, Germany) using full-wave time-domain solver. All measurements were done using a Magnetom™ “Terra” 7T scanner with an 8 channel parallel transmit (pTX) amplifier (Siemens, Erlangen, Germany). The pig hearts excised from the animals euthanized within approved by Local Authorities study ( approval # 55.2.DMS.2532-2-664) were used. The array testing included SNR, B1-shimming and g-factor mapping performed using vendor pulse sequences (“coil utility”). Imaging parameters were TR/TE=9/3.8ms, FA=150, voxel size=0.4x0.4x5mm, matrix= 256x256. The phantom comprised a plastic sphere ID=100 mm filled with tissue imitating sugar-salt solution (ε≈55). B1-shimming of the mTA was performed using scanner-side pTX B1-shimming platform. The comparison of SNR and g-factor maps with volume coil were done using a commercial 1TX/32RX head coil (Nova Medical, USA). Further assessment of PE-line reduction (acceleration) factor on image quality with the ex-vivo mTA was performed using an excised pig heart placed in a spherical container filled with 0.9% NaCl solution 3 hours post-excision. The assessment of acceleration factor (R) on the qualitative and quantitative imaging properties was performed using pulse sequences for T2* and diffusion tensor main vector components mapping. T2* times were measured using mGRE with TR=100ms, TE=2/4.6/5/15/20ms, voxel size=0.3x0.3x2mm, with R=1,2,3,4 using 24 reference lines for GRAPPA reconstruction. The relative differences of T2* were computed on pixel basis as:
Δi=[T2*(1) - T2*(i)] / T2*(1)
where, T2*(1) and T2*(i) denotes T2* computed at accelerations R=1 and R=2,3,4, respectively. DTI was performed using in-house stimulated-echo-based sequence with EPI-readout, with TR/TE=1000/41ms, matrix=128x104, FOV=243x300mm, R=2,3,4. Scanner reconstructed maps of DT main vector components were used for analysis.
Results
Figure 2A demonstrates the results of SNR-mapping for ex-vivo array performed before and after B1-shimmimg. The essential improvement of B1+-homogeneity (~80% lower SNR relative standard deviation) in the shimmed volume was easily observed.
The result of SNR-mapping for 1TX coil with histogram computed with the same volume is shown on Figure 2B. Even prior to B1-shimming the designed 8TX/16RX mTA demonstrates ~40% SNR gain in comparison to that obtained with the head coil.
Figure 3A shows the g-factor maps of head and mTA coils with histograms of marked volume. Figure 3B demonstrates severity of GRAPPA artefacts on the T2*-weighted images of myocardium acquired with both coils at high (R=4). Figure 4A shows T2*-maps acquired with ex-vivo array using accelerations R=1 to 4.
Figure 4B shows histograms of T2* maps and their relative differences for R=1..4. Figure 5A and B show maps and histograms of diffusion tensor main vector components acquired with R=2,3,4. The DTI maps and its histograms are highly consistent for R=2,3 and 4.
Discussion
The pTX-based B1-shimming employed for the novel ex-vivo mTA increases essentially mean value of SNR (on ≈20% ) and improves SNR-homogeneity approaching the one shown by the TX-birdcage of head coil. This effect observed for the dimension below the B1+ wavelength (~12 cm) proves high efficiency of the array in terms of both RF-design and geometry for using with available pTX-infrastructure. Despite of a factor 2 fewer number of elements, the g-factor of the mTA was found to be superior than that of the head coil with 32RX-array. In addition, no GRAPPA artifacts were observed with R=4, while with the conventional head coil these artifacts were well visible.Conclusion
A new pTX mTA demonstrated high TX and RX efficiency for (ultra)high ex-vivo myocardial tissue characterization imaging at 7T with essential superiority t 1TX commercial head coil.Acknowledgements
Financial support of German Ministry of Education and Research (BMBF grants: 01EO1004, 01E1O1504).
The hearts were kindly provided by Dr. Steffen Baltes for ex-vivo measurements after they were euthanized following approved use in project 55.2/DMS/2532-2-664 (Goverment of Low Franconia). Euthanasia was performed with intravenous application of 150mg/kg pentobarbital under isoflurane anesthesia with fentanyl analgesia.
We thank Siemens Healthineers for providing source code of diffusion imaging pulse sequences.
References
[1] Perrier, A. L., Grenier, D., Pouzin, A.,
et al., (2012). Design of a two-channel NMR coil using an impedance transformation
approach. IEEE Sensors Journal, 12(6), 1801-1808.
Figures

Figure 1
A. Design of the 8TX/16RX pTX array for ex-vivo studies of a myocardium tissue. Paring of individual elements for 8 channel TX configuration is shown by corresponding numbering.
B. Array shaping around spherical phantom /container for heart allocation. Elements are fixed on ABS 3D-printed elliptical housing.

Figure 2
A. SNR map of the Ex-vivo array before and after applying pTX-B1-shimming. The effect of the B1-homogeniety improvement by shimming is clearly seen in the zoomed region ( marked in rectangle) as well as on the histogram.
B. SNR map of the commercial 1Tx/32RX. The designed pTX-array demonstrates about factor 2 improvement of the SNR and B1-homogeneity comparable to the significantly volume single TX-coil with significantly large dimensions.

Figure 3
A. g-factors map of the Head and Ex-Vivo coils measured on 10mm sphere with tissue imitation solution. For the head coil the G-factors of acceleration R=3 and R=4 are significantly overlapped. The optimized shape and elements allocation of the ex-vivo array allows for significantly lower g-factors mean value and improved homogeneity and, thus, minimized noise amplification in accelerated parallel imaging regimes.
B. Images of an ex-vivo myocardial slice acquired using non-accelerated and R=4 accelerated MRI. The accelerated image is essentially artefact-free whereas significant GRAPPA-reconstruction artefacts appear on head coil images due to less optimal g-factor.
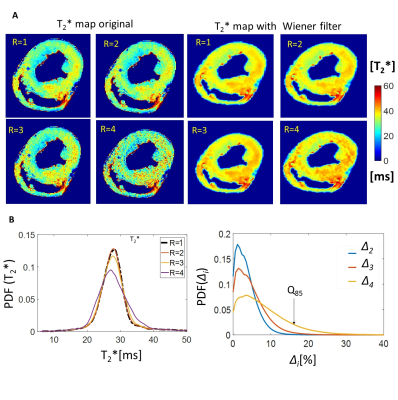
Figure 4 A.
High resolution T2* maps of the myocardium tissue ex-vivo acquired with different parallel imaging acceleration factors. The optimized g-factor allows for acquiring non-distorted T2* maps with up to R=3 acceleration. The increased noise penalty at R=4 acceleration leads to only minimal distortions in the T2* spatial distribution which could be neutralized by noise-reduction filters. Quantitative analysis of the acceleration induced distortions in the high resolution T2* maps. Histograms shape remains well preserved for R=2,3. The pixel-based ground-truth relative difference ∆ for R=4 remains within 15% (~85% of pixel values).
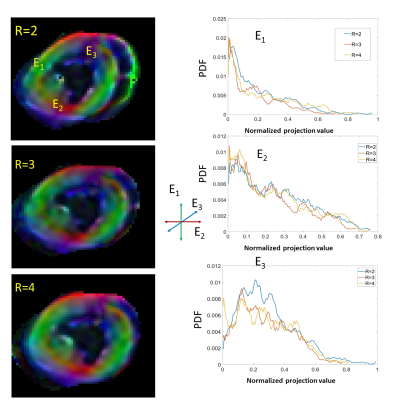
Figure 5
Analysis of the parallel imaging acceleration effect on the consistency of the DTI maps of the myocardial tissue acquired with 2.3x2.3x3mm voxel size. The distribution of components of the main vector of diffusion tensor remain preserved with R=2,3,4 acceleration factors. The variation in histogram is only slightly increased for the third component (E3) (basal-apical direction in the heart) transversal to the imaging plane.