1457
Development of Asymmetric 8Tx/16Rx Coil Array for Human Cardiac MRI at 7 T: RF Shimming and SAR Safety Study1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
A novel transceiver 8Tx/16Rx coil array was simulated and developed for cardiac magnetic resonance imaging (cMRI) in humans at 7T. The developed cardiac coil array consists of two independent parts, the semi-curved anterior array and the flat posterior array, each array composed of identical 8-elements. An optimization routine has been developed in MATLAB to find the optimal phases for B1+-field homogeneity with minimal local SAR values within the Duke and Ella human models. RF-shimming improves the B1+-field homogeneity by 30% and 46% for Duke and Ella, respectively. The novel cardiac pTx array has higher Tx-efficiency and flexibility in RF-shimming compared to the 1Tx commercial coil.
Introduction
MRI at ultrahigh field (UHF) is a popular imaging modality to improve the signal-to-noise ratio (SNR) compared to conventional clinical MRI at 3T and 1.5T. However at 7T, the RF-wavelength (λ≈12 cm) within the object to be imaged such as heart is becoming comparable to the object’s dimension which results in B1+-field inhomogeneity. A number of engineering challenges hamper the development of an optimized pTx-coil array for cMRI at 7T. First, the heart’s position is in the middle of the thorax with about 6-cm distance from the coil surface. Second, the heart is surrounded by inhomogeneous tissues such as the lung which produces dielectric resonances resulting in stronger B1+-field inhomogeneity at the heart-lung tissue interface. Nevertheless, several MR groups successfully performed human body imaging at UHF using different types of coils including, multichannel loops 1-4 and dipole antenna arrays 5-7. Optimization of the B1+-field inhomogeneity for cMRI 8-10 was considered a challenge until novel RF-coils were developed. The purpose of this study was to develop 8Tx/16Rx coil array for human cMRI at 7T.Materials and Methods
A novel asymmetric 8Tx/16Rx coil array was developed for human cMRI at 7T. Both anterior and posterior arrays are composed of identical 8-elements. The size of the central two-elements 1 and 2 are 4.5×9.8 cm2. The decoupling between the central two-elements was accomplished using a common conductor and shared decoupling capacitor (C1d) 11. The other six-elements were distributed around the central two-elements in a reversed way to allow good decoupling between all elements and varying B1+-field distributions. The size of elements 3 and 4 was 3.5×9 cm2. Elements 3 and 4 were decoupled with elements 1 and 2 using the shared decoupling capacitor (C2d). The elements 5 and 7 and the reversed identical elements 6 and 8 were decoupled using the shared decoupling capacitor (C3d). The sizes of the elements 5 and 6 and the elements 7 and 8 are 6.6×6.6 cm2 and 6.6×7 cm2, respectively. The elements 1, 2, 3 and 4 were decoupled from the neighboring elements (5, 6, 7 and 8) using capacitive decoupling (C4d, C5d, C6d and C7d) in addition to decoupling gaps of 1.4 and 2cm. The total external dimension for the array was 20.8×24.1 cm2 (Figure 1). The copper track width is 4mm etched on a 0.3mm FR4-PCB. For specific absorption rate (SAR) safety and B1+-field optimization, EM-simulations were performed using CST-Microwave-Studio. RF-circuit co-simulation was employed for perfect matching, tuning, and decoupling at 297.2-MHz 12. The coil was loaded with Duke and Ella human models with 2×2×2mm3 resolution. The distance between the anterior array and the human models is 8mm and 10mm from the posterior array. The final total numbers of mesh cells are 64.9 and 56.9-million for Duke and Ella, respectively. The local averaged 10g SAR values were evaluated in CST-MWS using IEEE/IEC-62704-1 standard averaging method. The coil was excited with the same input power (Pin=8W). A routine was developed in MATLAB to homogenize the B1+-field within the heart of a ROI of 8×8×6cm3. The optimization target function was designed to provide a minimum relative standard deviation (RSD)=SD(B1+)/mean(B1+), maximum transmission efficiency (Tx, eff)=mean(B1+)/√(Pin), and maximum Figure-of-Merit (FoM)= mean(B1+)/(RSD*√(max(SAR10g))) within the ROI. All measurements were performed on a 7T whole-body MAGNETOM Siemens Terra scanner equipped with a 16-kW RF amplifier for 8-channel pTx-mode. The commercial MRI-Tools coil was tested in 1Tx-mode 4.Results and Discussion
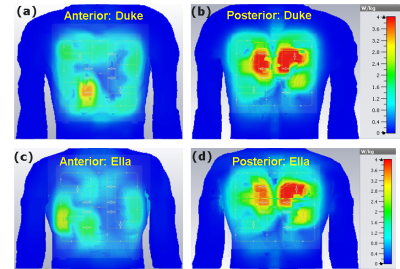
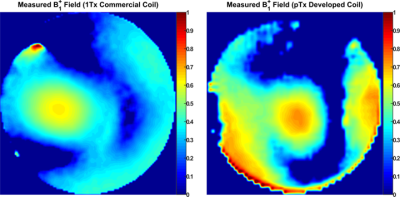
Figure 2 displays the simulated distribution of the central axial B1+-field within the Duke and Ella human models before and after optimization. With zero phases, the RSD and FOM were evaluated as 0.40 and 0.37 for Duke and 0.39 and 0.49 for Ella. RF-shimming improves the B1+-field homogeneity by 30% and 46% for Duke and Ella, respectively. The FoM was improved by 54% for Duke. Highly homogeneous B1+-field distribution was obtained for Ella with about 2-times improvement in the FoM compared to Duke. RF-shimming improves the Tx-efficiency by 20% and 46% for Duke and Ella, respectively. With zero phases, the maximum 10 g SAR values were found to be 4.81 and 3.97 W/kg for Duke and Ella, respectively (Figure 3). After optimization, the maximum 10g SAR values are 4.8 and 4.43 W/kg for Duke and Ella, respectively. The highest peak local SAR is pronounced near to the posterior array. Even without pTX optimization, the developed coil has focused B1+-field at center of the phantom with better Tx-efficiency compared to the MRI-Tools commercial coil (Figure 4).Conclusion
A novel 8Tx/16Rx cardiac coil array has been developed at 7T. EM-simulations were performed for a novel 8Tx/16Rx cardiac array to establish the robust and reliable procedures for pTx optimization of the B1+-field and local SAR in the Duke and Ella human models.Acknowledgements
Financial support: German Ministry of Education and Research (BMBF, grants: 01EO1004, 01E1O1504).References
- Graessl, A., Renz, W., Hezel, F., et al., (2014). Modular 32-channel transceiver coil array for cardiac MRI at 7.0 T. Magnetic resonance in medicine, 72(1), 276-290.
- Dieringer, M. A., Renz, W., Lindel, T., et al., (2011). Design and application of a four-channel transmit/receive surface coil for functional cardiac imaging at 7T. Journal of Magnetic Resonance Imaging, 33(3), 736-741.
- Gräßl, A., Winter, L., Thalhammer, C., et al., (2013). Design, evaluation and application of an eight channel transmit/receive coil array for cardiac MRI at 7.0 T. European journal of radiology, 82(5), 752-759.
- Thalhammer, C., Renz, W., Winter, L., et al., (2012). Two-dimensional sixteen channel transmit/receive coil array for cardiac MRI at 7.0 T: design, evaluation, and application. Journal of Magnetic Resonance Imaging, 36(4), 847-857.
- Oezerdem, C., Winter, L., Graessl, A., et al., (2016). 16-channel bow tie antenna transceiver array for cardiac MR at 7.0 tesla. Magnetic resonance in medicine, 75(6), 2553-2565.
- Raaijmakers, A. J. E., Luijten, P. R., and van Den Berg, C. A. (2016). Dipole antennas for ultrahigh-field body imaging: a comparison with loop coils. NMR in Biomedicine, 29(9), 1122-1130.
- Ertürk, M. A., Wu, X., Eryaman, Y., Van de Moortele, P. F., et al., (2017). Toward imaging the body at 10.5 tesla. Magnetic resonance in medicine, 77(1), 434-443.
- Xin, S. X., Huang, Q., Gao, Y., et al., (2013). Fetus MRI at 7 T: Shimming Strategy and SAR safety implications. IEEE Transactions on Microwave Theory and Techniques, 61, 2146-2152.
- Ibrahim, T. S. (2006). Ultrahigh-field MRI whole-slice and localized RF field excitations using the same RF transmit array. IEEE transactions on medical imaging, 25(10), 1341-1347.
- Mao, W., Smith, M. B., and Collins, C. M. (2006). Exploring the limits of RF shimming for high-field MRI of the human head. Magnetic Resonance in Medicine, 56(4), 918-922.
- Perrier, A. L., Grenier, D., Pouzin, A., et al., (2012). Design of a two-channel NMR coil using an impedance transformation approach. IEEE Sensors Journal, 12(6), 1801-1808.
- Kozlov, M., and Turner, R. (2009). Fast MRI coil analysis based on 3-D electromagnetic and RF circuit co-simulation. Journal of magnetic resonance, 200(1), 147-152.
Figures