1310
BEES Knees: Bilateral Expedited Exam Supporting Quantitative Imaging of Knees1Radiology, UCSF, San Francisco, CA, United States
Synopsis
Osteoarthritis (OA) is a degenerative joint disease that commonly affects bilateral joints. This study combined simultaneous bilateral knee MRI with an automatic image processing for faster acquisition to image biomarker extraction. Simultaneous bilateral knee MRIs of 5 healthy volunteers was compared to singularly acquired knee images. Isotropic 3D CUBE FSE were used to automatically segment cartilage. Voxel based relaxometry from 3D combined T1ρ/T2 was evaluated for both types of acquisition. Our results show the ability of dual coil configuration allows for high resolution isotropic images, while also retaining the accuracy of quantitative data when compared to singly acquired bilateral knees.
Introduction
Osteoarthritis (OA) is a commonly bilateral degenerative joint disease1,2. Studies have shown the effectiveness of MRI to assess morphological changes and detect early biochemical changes in the knee3, 4, 5, however with long scan times, MRI has been a deterrent for bilateral joint screening. Kogan et al. demonstrated reduced scan time and comparable image quality with simultaneous knee MRI6. This study further aims to combine simultaneous bilateral knee acquisition with automatic image post-processing for faster, end-to-end quantitative pipelines, from acquisition to image biomarker extraction. Automatic cartilage segmentation and morphometry from 3D CUBE, and voxel based relaxometry from 3D combined T1ρ/T2 will be evaluated7.Methods
Five (4 females) healthy volunteers were recruited for this study and imaged on a 3T MR750 GE scanner. Subjects were positioned supine, feet first with two medium flex, receive only coils wrapped on each knee. 3D CUBEs were acquired for all subjects, and 3D T1ρ/T2 on 4.
Image Acquisition and hardware set up: Coil modifications and set up for this study were as described by Kogan et al. The MR acquisition of bilateral knees using two coil configuration included: 1) 3D high resolution isotropic CUBE FSE with fat suppression and 2) 3D T1ρ/T2. The 3D T1ρ/T2 sequence was also acquired to image both knees with single coil configuration for comparison with synchronous bilateral acquisition. MR acquisition parameters are summarized in Table 1. B1 mapping was performed by using Bloch-Seigert shift method, and B0 off-resonance map was measured by acquiring six echoes for inhomogeneity visualization8,9. To ensure proper fat suppression, left and right knee shim and center frequency values were averaged for all simultaneous bilateral knee acquisition.
Image post processing and analysis: Line of profile analysis was used to automatically detect central noise point to split the two knees that were acquired simultaneously.
3D T1ρ/T2: T1ρ and T2 maps were obtained by fitting the images with different TSL and TE values, applied to each voxel as described by Pedoia et al10. Six cartilage regions were semi-automatically segmented on simultaneously acquired knees and singly imaged knees were non-rigidly registered to their simultaneous imaged counterpart10. Mean T1ρ and T2 values were computed for each compartment for comparison between single and dual knee acquisitions.
3D CUBE: High resolution cartilage and meniscus segmentation was inferred from a previously trained deep learning model (3D V-net11). Training and validation was performed on 480 manually segmented 3D CUBE FSE acquired with classical single knee protocol. No additional training on data acquired with the current simultaneous bilateral knee protocol was done and no model fine tuning was performed. After segmentation, left and right knees were registered in the same space using Iterative Closest Point algorithm (ICP) and local differences in cartilage thickness between both knees were evaluated and rendered in 3D color map for visual inspection.
Results
B0 and B1 maps showed inhomogeneity in certain regions for the dual coil configuration. Figure 1 shows a flip angle scale map from 0.7 to 1.3 for the B1 and B0 ranging from -100 to 50Hz. Averaged shim and center frequency technique resulted in good fat suppression as seen in Figure 2.
Figure 3 shows an example of the results of our fully automatic pipeline for the evaluation of bilateral morphological difference in cartilage surface. Figure 3A shows a medial slice in left and right knees, acquired simultaneously, exhibiting cartilage thickness differences between the knees, quantitatively evaluated and visualized by our pipeline. Figure 3B shows localized difference in the trochlea, which are difficult to detect when only relying on 2D inspection, due to a limited perspective.
Figure 4 demonstrates T1ρ maps of cartilage regions of a knee acquired with dual and single coil configurations. The difference between the dual and singly acquired knee compartments were 36.19±3.34 and 35.94±3.87ms (p=0.57) for T1ρ and 27.57±2.6 and 27.87±3.33ms (p=0.47) for T2 relaxation. The correlation between the two acquisitions is shown in Figure 5 with a Bland-Altman plot.
Discussion & Conclusion
This study demonstrates the ability of dual coil configuration for high resolution isotropic images, while also retaining the accuracy of quantitative data when compared to singly acquired bilateral knees. Our automatic segmentation model did not require further training since the data it was applied to was similar to the trained data, but better quality. This fully automated pipeline allows for 3D visualization of morphological abnormalities, which are sometimes difficult to see in a 2D perspective. With scan times under thirty minutes, quantitative accuracy to single acquisitions and faster start to finish image processing, simultaneous bilateral joint imaging with MRI is a promising tool for OA screening.Acknowledgements
We would like to thank Thomas Link, MD, PhD for reviewing the quality of high resolution 3D CUBE FSE images.
This project was supported by NIH-NIAMS grant P50AR060752 (SM).
References
- R. C. Lawrence, D. T. Felson, C. G. Helmick, L. M. Arnold, H. Choi, R. A. Deyo, S. Gabriel, R. Hirsch, M. C. Hochberg, G. G. Hunder, J. M. Jordan, J. N. Katz, H. M. Kremers, F. Wolfe, and Workgroup National Arthritis Data, "Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II," Arthritis Rheum 58(1), 26-35 (2008).
- A. J. Metcalfe, M. L. Andersson, R. Goodfellow, and C. A. Thorstensson, "Is knee osteoarthritis a symmetrical disease? Analysis of a 12 year prospective cohort study," BMC musculoskeletal disorders 13, 153 (2012).
- C. G. Peterfy, A. Guermazi, S. Zaim, P. F. Tirman, Y. Miaux, D. White, M. Kothari, Y. Lu, K. Fye, S. Zhao, and H. K. Genant, "Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis," Osteoarthritis Cartilage 12(3), 177-190 (2004).
- E. David-Vaudey, S. Ghosh, M. Ries, and S. Majumdar, "T2 relaxation time measurements in osteoarthritis," Magn Reson Imaging 22(5), 673-682 (2004).
- A. J. Wheaton, F. L. Casey, A. J. Gougoutas, G. R. Dodge, A. Borthakur, J. H. Lonner, H. R. Schumacher, and R. Reddy, "Correlation of T1rho with fixed charge density in cartilage," J Magn Reson Imaging 20(3), 519-525 (2004).
- F. Kogan, E. Levine, A. S. Chaudhari, U. D. Monu, K. Epperson, E. H. G. Oei, G. E. Gold, and B. A. Hargreaves, "Simultaneous bilateral-knee MR imaging," Magn Reson Med 80(2), 529-537 (2018).
- X. Li, E. T. Han, R. F. Busse, and S. Majumdar, "In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS)," Magn Reson Med 59(2), 298-307 (2008).
- Laura I. Sacolick, Florian Wiesinger, Ileana Hancu, and Mika W. Vogel, "B1 mapping by Bloch-Siegert shift," 63(5), 1315-1322 (2010).
- H. Yu, A. Shimakawa, C. A. McKenzie, E. Brodsky, J. H. Brittain, and S. B. Reeder, "Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling," Magn Reson Med 60(5), 1122-1134 (2008).
- V. Pedoia, X. Li, F. Su, N. Calixto, and S. Majumdar, "Fully automatic analysis of the knee articular cartilage T1rho relaxation time using voxel-based relaxometry," J Magn Reson Imaging43(4), 970-980 (2016).
- Milletari F, Navab N, Ahmadi SA. V-Net: Fully Convolutional Neural Networks for Volumetric Medical Image Segmentation. 2016. https://arxiv.org/abs/1606.04797. Accessed November 6, 2018.
Figures




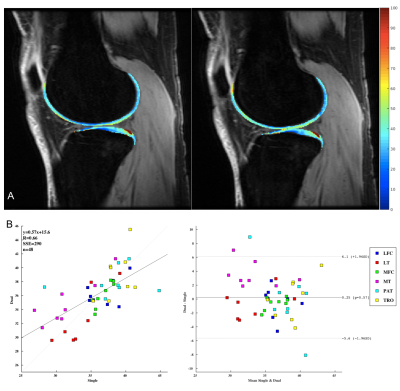
Figure 5. Six cartilage regions were lateral femoral condyle (LFC), medial femoral condyle (MFC), lateral tibia (LT), medial tibia (MT), patella (P), and trochlea (TRO) were semi-automatically segmented; A) Demonstrates T1ρ maps of LFC, LT, TRO of left knee (left) acquired singularly same knee acquired in a simultaneous dual coil configuration (right), showing similar spatial distribution; B) Bland-Altman plot showcasing a correlation between T1p values for knees acquired simultaneously with a dual coil configuration and knees acquired with a single coil configuration.