1266
Whole-Body Mapping of Spontaneous Mechanical Activities in Musculature1Section on Experimental Radiology, Department of Diagnostic and Interventional Radiology, University Hospital of Tübingen, Tübingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 3School of Biomedical Engineering & Imaging Sciences, King’s College London, St. Thomas’ Hospital, London, United Kingdom, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Whole-body quantification of spontaneous mechanical activities is of high interest for the assessment of the activity distribution in healthy and non-healthy population. Therefore, a measurement protocol and spatial mapping is investigated for accurate quantification of small subtle spontaneous activities in the human skeletal musculature over the whole-body. This work enables to assess spontaneous activity in muscular regions which are important for potential evaluation and grading in neuromuscular disorders.
Introduction:
Spontaneous mechanical activities in musculature (SMAM1) are recorded by diffusion-weighted imaging (DWI) of the resting human skeletal musculature. Previously published studies2,3,4 evaluated the electromyographic2 and anatomical3 correlation as well as spatial extension4 with restriction to the right lower leg. On the other hand, patients with neuromuscular disorders might show fasciculations and fibrillations in other muscular areas as revealed by needle electromyography or ultrasound imaging.
In this work, the feasibility to image and detect spontaneous muscular activities in different areas of the entire body is investigated to overcome the limitations of previous studies. Moreover, the presence of spontaneous activities in other skeletal muscles than the lower leg is verified. Results were spatially mapped to enable direct comparison of the whole-body activity of individual subjects.
Methods:
Whole-body data sets were acquired under free-breathing conditions from three healthy volunteers (age: 25.3±2.3 years, BMI: 26.7±2.5 kg/m²). Measurements were conducted on a 3 T MR scanner (MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany) with a prototype diffusion-weighted stimulated-echo simultaneous-multi-slice (DW-STE-SMS) EPI sequence. A schematic illustration of the whole-body multi-bed acquisition protocol is given in Fig. 1. Sequence parameters: matrix size: 160 × 90, FoV: 480 × 270 mm², TE: 34 ms, TR: 1000 ms, slice-thickness: 6 mm, number of simultaneously excited slices: 2, number of slices in slab: 4, slice-distance: 800 %, b-value: 100 s/mm², number of repetitions per bed position: 200, bed positions: 6. The mixing time TM was set to 25 ms to suppress signal voids based on respiratory motion in the thoracic and abdominal regions. Total measuring time (for whole-body assessment) was approx. 21 min (3:24 min per bed position). A stimulated-echo excitation was chosen to increase the sensitivity of the MR sequence on spontaneous muscular activities due to the prolonged diffusion-sensitive time in comparison to spin-echo excitation5.
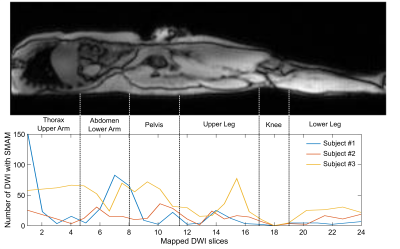
Since DWI measurements were conducted under free-breathing conditions, spatial accordance between single DWI in each data set was ensured by image registration. Therefore, all images in a data set are registered on a reference image, which was calculated from principle-component analysis and subsequently registered by a Local-All-Pass6,7 registration. Gross movements and signal voids originating from breathing (intercostal muscles and diaphragm) were manually excluded to ensure accurate results. Data sets were semi-automatically analyzed by a graph-based segmentation approach8 and visually revised to ensure reliability. Activity was analyzed in terms of the number of SMAM-affected DWI and event count maps (ECM). Distribution is mapped to the same common space by localization of prominent body land marks (femoral head, knee) and heuristic approaches9.
Results & Discussion:
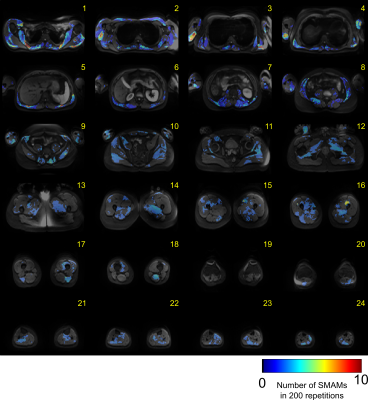
Averaged DWI slices are depicted in Fig. 2 showing sufficient image quality for assessment of signal voids due to spontaneous muscular activity across the whole-body. Small signal decays and background signal inhomogeneity were visible at the thigh and upper-arm musculature. Nevertheless, evaluation of spontaneous muscular activity is still possible in these regions due to sufficient signal intensity. ECMs of Subject #1 are illustrated in Fig. 3. Distinct activity was measured at the musculus psoas major in slice #7 and #8. No activity was measured in the muscular region of the arms as well as only moderate activity in the leg musculature. In contrast, Subject #3 (Fig. 4) shows clear activity in muscles of the thigh and upper arm. However, activity in the musculus psoas major is reduced for this subject. Mapping of the whole-body activity is given in Fig. 5 showing clear differences between all three subjects. Subject #3 shows a raised number of SMAMs in contrast to Subject #1 and #2. The previously mentioned activities in thigh and psoas muscles are clearly visible as activity peaks in the spatial mapping. Visual inspection of DWI data sets showed that SMAMs did not occur simultaneously in the left and right extremity.Conclusion:
The proposed whole-body protocol enables accurate whole-body quantification of small spontaneous mechanical activities. Spontaneous activity was detected in several skeletal muscles in all body areas verifying that these activities are not restricted to the human lower leg. Distribution of activities over the whole-body differs in the three subjects examined. Furthermore, the work demonstrates the feasibility of measuring spontaneous activity in all major muscular regions which are important for potential examination and grading in neuromuscular disorders.Acknowledgements
This work was supported and funded by the German Research Foundation (DFG) under Grants SCHI 498/11‐1 and YA 28/16‐1.References
[1]: Steidle G, Schick F. Addressing spontaneous signal voids in repetitive single-shot DWI of musculature: spatial and temporal patterns in the calves of healthy volunteers and consideration of unintended muscle activities as underlying mechanism. NMR Biomed 2015;28(7):801-10.
[2]: Schwartz M, Steidle G, Martirosian P, Ramos-Murguialday A, Preißl H, Stemmer A, Yang B, Schick F. Spontaneous mechanical and electrical activities of human calf musculature at rest assessed by repetitive single-shot diffusion-weighted MRI and simultaneous surface electromyography. Magn Reson Med 2018 May;79(5):2784-2794. doi: 10.1002/mrm.26921.
[3]: Schwartz M, Martirosian P, Steidle G, Erb M, Stemmer A, Yang B, Schick F. Volumetric assessment of spontaneous mechanical activities by simultaneous multi-slice MRI techniques with correlation to muscle fiber orientation. NMR Biomed 2018 Nov;31(11):e3959. doi: 10.1002/nbm.3959.
[4]: Schwartz M, Steidle G, Fallah F, Martirosian P, Schmidt H, Schick F. Automated detection of signal voids in different muscle groups of the leg – A means to analyse spontaneous muscular activity from time series of diffusion weighted MR images. Proceedings of the 32nd Annual Scientific Meeting ESMRMB 2015, October 2015, Edinburgh, United Kingdom.
[5]: Schwartz M, Steidle G, Martirosian P, Ramos-Murguialday A, Stemmer A, Yang B, Schick F. Estimation of the Sensitivity Characteristics and Detection Capability of Diffusion-Weighted MR Sequences in Imaging Spontaneous Mechanical Activity in Musculature. Proceedings of the Annual Meeting ISMRM 2017, April 2017, Honolulu, USA.
[6]: Gilliam C, Küstner T, Blu T. 3D Motion Flow Estimation using Local All-Pass Filters. Proc. IEEE Int. Symp. Biomed. Imag. (ISBI) 2016, April 2016, Prague, Czech Republic.
[7]: Küstner T, Neumann V, Schwartz M, Würslin C, Martirosian P, Gatidis S, Schwenzer NF, Schick F, Yang B, Schmidt H. An MR Motion Correction toolbox for registration and evaluation. Proceedings of the Annual Meeting ISMRM 2016, May 2016, Singapore.
[8]: Schwartz M, Steidle G, Martirosian P, Yang B, Schick F. Graph-based segmentation of signal voids in time series of diffusion-weighted images of musculature in the human lower leg. Proceedings of the Annual Meeting ISMRM 2016, May 2016, Singapore.
[9]: Würslin C, Machann J, Rempp H, Claussen C, Yang B, Schick F. Topography mapping of whole body adipose tissue using a fully automated and standardized procedure. J Magn Reson Imaging 2010;31(2):430-9.
Figures