1220
Retrospectively Gated UTE MRI with Deep-Learning Segmentation to Quantify Preclinical Lung Fibrosis Severity1Department of Biomedical Engineering, University of Cincinnati, Cincinnati, OH, United States, 2Center for Pulmonary Imaging, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 3Molecular & Developmental Biology Graduate Program, Cincinnati, OH, United States, 4Division of Neonatology and Pulmonary Biology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 5Division of Pulmonary and Critical Care, University of Kansas Medical Center, Kansas City, KS, United States
Synopsis
Fibrosis contributes to morbidity and mortality in an array of pulmonary diseases, and no therapies exist to halt or reverse its progression. This paucity of treatment strategies results from poorly understood etiology, making it essential to develop animal models that fully mimic human disease and identify noninvasive, quantitative markers for fibrosis severity for use in animal models and patients. Here we report an image reconstruction and analysis pipeline, combining retrospectively gated ultrashort echo time (UTE) MRI and deep-learning segmentation to quantify lung fibrosis progression in Surfactant Protein C (SP-C) knock-in models based on mutations that predispose humans to lung fibrosis.
Introduction
The mechanisms underlying pulmonary fibrosis initiation and progression are poorly understood, resulting in an absence of effective therapies. Inadequate understanding of disease etiology results from the lack of: 1) animal models that capture fibrotic lung disease biology and 2) effective methods to quantify disease progression in vivo. To this end, UTE MRI—despite physiological motion and rapid T2* relaxation (~0.4ms at 7T)—has shown promise in quantifying lung disease[1-5]. Here we extend the utility of UTE by applying it to two novel and biological relevant mouse models based on SP-C mutations that predispose humans to lung fibrosis[8]. Further, based on an elliptical UTE sequence, we developed an image reconstruction and analysis pipeline that eliminated respiratory motion through retrospective-gating, allowing fibrotic burden to be quantified[3,5]. Data generated using this pipeline also provided the training data needed to enable fully automated, deep-learning segmentation throughout fibrosis progression.Methods
Model:
Two knock-in mutations—L184Q (LQ, equivalent to the L188Q mutation responsible for familial fibrosis in humans[8]) and I73T (IT, the most common SP-C mutation associated with lung fibrosis)[6]—along with wildtype and wildtype/mutant heterozygotes were studied. Mice (N=20; 4 WT/WT; 4 WT/IT; 4 IT/IT; 4 WT/LQ; 4 LQ/LQ) were treated every 2 weeks over 8-weeks with a small dose (1U/kg) of bleomycin to induce minor inflammatory lung injury. Mice were imaged prior to treatment and following injury before being sacrificed for histology on Days 160 (N=10) and 261 (N=10).
MRI:
Ellipsoidal UTE images were acquired[1] using a Bruker BioSpin 7T. Imaging parameters included: FOV=32x32x64mm; matrix=1283; BW=277kHz; TE/TR=0.080/6.0ms; α=5.3°; views=44,334; dummy-cycles=300. Respiratory-gated images were reconstructed by binning radial data based on small respiratory-based intensity fluctuations at k-zero (i.e., first point on each FID)[3].
Image Quantification:
The thoracic cavity was manually segmented at end-expiration in Amira (Figure 1), and regions of interest were drawn in the heart to normalize lung-signal intensity. A high-signal threshold based on distribution of weighted-signal from healthy parenchyma (i.e., all animals pre-treatment) was developed to differentiate low-signal parenchyma from high-signal vasculature and fibrotic tissue (Figure 2). High-signal volume was defined as voxels with weighted signal > mean+3σ. Whole-lung masks served as training data (Figure 1) for a 3D convolutional neural network (CNN). The CNN architecture was modified based off the popular 2D Unet segmentation model[7], to accommodate 3D images after reshaping to 128x128x64. Model training was performed on a MacPro with 6-processing cores using a NVIDIA Titan Xp graphic processing unit (GPU).
Results
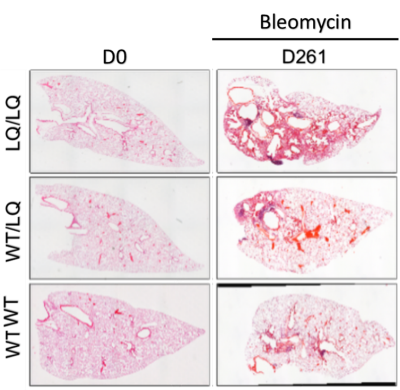
Minimal injury was observed in WT/WT mice via conventional histology (Figure 3), whereas intermediate disease was seen in heterozygotes and sustained fibrosis in homozygotes. Specifically, LQ/LQ and IT/IT mice displayed progressive fibrotic injury during treatment. Moreover, the LQ/LQ mice displayed the most significant fibrotic burden and showed significant fibrosis even after 255 days.
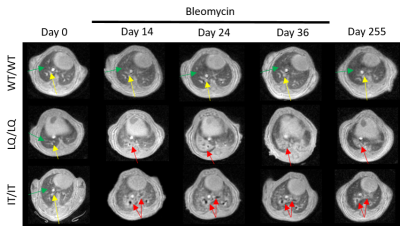
Using radial UTE MRI and retrospective-gating protocol, motion-artifact-free images were successfully reconstructed at inspiration and expiration for all genotypes at all time-points. Injury-associated, high-signal intensity volume was observed in lung images of all treated animals, qualitatively matching histological observations. (Figure 4). Moreover, the high-signal threshold of mean+3σ quantitatively separated healthy parenchyma from vasculature and fibrotic tissue at all time-points. In all genotypes, signal intensity was the highest after first dose of bleomycin, resulting in an ~13-43% increase in high-signal volume (Figure 5). As treatment continued, lungs developed persistent fibrosis over time, which matched that of histology (Figure 3).
Manual segmentations typically required 30-45 minutes depending on fibrosis severity. However, by training the CNN with manually segmented lung masks at end-expiration, we were able to automatically segment the thoracic cavity, reducing segmentation time ~200-fold. Moreover, we found that, despite being trained on expiratory images only, the CNN segmentation could be readily applied to the highly undersampled images reconstructed from inspiratory data (Figure 1).
Discussion and Conclusions
Biological realistic behavior, similar to that observed in human disease, was observed in the LQ/LQ mice, and this behavior matched with retrospectively gated UTE. Specifically, the LQ/LQ mutant mice displayed progressive and ultimately persistent fibrosis, a feature that has not been observed previously in any mouse model of lung fibrosis based on mild injury. Additionally, the deep-learning algorithm was able to segment images obtained at end-inspiration, despite being training on only expiratory images, suggesting that physiological parameters such as tidal volume and minute ventilation can be calculated by combining this segmentation approach with retrospective-gating. Together, these results demonstrates that we have developed 1) a unique and biologically relevant mouse model that captures key elements of human disease and 2) a robust reconstruction and analysis pipeline for quantifying lung fibrosis burden that will likely be useful is assessing therapy response.Acknowledgements
The authors wish to thank Scott Dunn for assisting with data collection and the NVIDIA Corporation for the donation of a Titan Xp GPU.
Supporting Grants: NIH R01HL103923 and R01HL129747 (T.E.W.), R00HL111217 (Z.I.C.), and the Cincinnati Children’s Hospital Medical Center, Center for Translational Fibrosis Research
References
1. Guo J, Cao X, Cleveland ZI, Woods JC. Murine pulmonary imaging at 7T: T2* and T1 with anisotropic UTE. Magnetic Resonance in Medicine 2017:DOI: 10.1002/mrm.26872.
2. Guo J, Cleveland ZI, Hardie WD, Davidson C, Xu X, Woods JC. "Quantifying tidal volume and pulmonary fibrosis in a TGF-α transgenic mouse model with retrospective self-gating UTE MRI". International Society for Magnetic Resonance in Medicine 25th Annual Scientific Meeting, Honolulu, HI, USA, April 22 - 27, 2017
3. Guo J, Cao X, Cleveland ZI, Woods JC. "Retrospective self-gated 3D UTE MRI in mouse lung". International Society for Magnetic Resonance in Medicine 24th Annual Scientific Meeting, Singapore, May 07 - 13, 2016.
4. Guo J, Cao X, Cleveland ZI, Woods JC. "Anisotropic-resolution, quantitative pulmonary UTE MRI by ellipsoidal k-space coverage". 57thExperimental Nuclear Magnetic Resonance Conference, Wyndham Grand Hotel, Pittsburgh, Pennsylvania, April 10 - 15, 2016.
5. Higano NS, Hahn AD, Tkach JA, Cao XF, Walkup LL, Thomen RP, Merhar SL, Kingma PS, Fain SB, Woods JC. Retrospective Respiratory Self-Gating and Removal of Bulk Motion in Pulmonary UTE MRI of Neonates and Adults. Magnetic Resonance in Medicine 2017; 77(3):1284-1295.
6. Nureki, S.-I., et al. (2018). "Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis." The Journal of Clinical Investigation 128(9): 4008-4024.
7. Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for bio- medical image segmentation. Proc Int Conf Med Image Comput Com- put-Assist Interv 2015; 9351:234–241.
8. Thomas AQ, Lane K, Phillips J, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, Roberts R, Haines J, Stahlman M, Loyd JE. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. American Journal of Respiratory and Critical Care Medicine 2002; 165(9):1322-1328.
Figures