1196
Feasibility of brain pathology assessment with diffusion imaging on a portable scanner using a fixed encoding field1A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 4A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, 02129, MA, United States, 5Harvard Medical School, Cambridge, MA, United States
Synopsis
Diffusion-weighted MRI is widely used for diagnosing brain pathology, but is typically not available close to the site of acute brain injury due to size, weight, power, and exclusion-zone constraints. Recently-developed lightweight brain scanners have achieved portability by using inhomogeneous B0 fields and by correcting for field imperfections during image reconstruction. Building on work done by the petroleum industry with single-sided borehole magnets, we use the built-in spatial encoding field of a portable brain scanner for both diffusion-encoding and spatial-encoding. We also introduce a method for using dummy refocusing pulses to vary the b-value without changing TEeff and T2-contrast.
Introduction
Diffusion-weighted MRI (DWI) is an indispensable tool for diagnosing and staging a wide range of brain pathology including cerebral infarcts [1], infections, edema and trauma. Extending this capability closer to the site of acute injury, such as ambulances and remote treatment facilities, could enable faster intervention and improved care. However, conventional MRI scanners are poorly-suited to this application due to size, weight, power, and exclusion-zone constraints. One way to improve portability is to allow the main magnetic field to be inhomogeneous [2, 3], but this raises challenges for performing diffusion-weighted imaging. The petroleum-prospecting industry has previously shown diffusion profiling of rock porosity and fluid content using the “always-on” field gradients surrounding single-sided magnets that are lowered down bore-holes [4].
Building on this work, we show proof-of-concept diffusion-weighted phantom images acquired in a ~100kg brain scanner prototype based on an 80mT permanent magnet Halbach-array [5]. The magnet achieves light-weight and portability by allowing the main magnetic field to be highly-inhomogeneous (ΔB0/B0~1%) and by accounting for the field variations during generalized image reconstruction. Because the magnet does not require exclusion zones, cryogens, or industrial power, it is well-suited to acute brain injury diagnosis in settings such as an ambulance. The magnet was designed to have a built-in readout-encoding field with approximately linear variation across the FOV (~7.5mT/m) [5]. Using a modified CPMG pulse sequence, we show that this built-in field can also be used for diffusion-encoding with clinically-relevant b-values. We also show a new method for using dummy refocusing pulses during the diffusion-encoding module to vary the b-value without changing TEeff or T2-contrast.
Methods
Figure 1 shows the magnet and gradient coil hardware used for DWI experiments. Previously-shown 2D and 3D T2-weighted images are shown for reference [6]. Figure 2 shows the modified-CPMG spin-echo sequence that uses the built-in ΔB0-field for readout and the Gz gradient coil for phase encoding. The readout-field nonlinearity is small enough over the FOV (7cm) to permit simple 2D-FFT image reconstruction.
Frequency-swept RF pulses are used to excite and refocus spins across the wide bandwidth of the inhomogeneous B0field (~100 KHz) [7]. During the diffusion-encoding module, dummy refocusing pulses are added to change the encoding time, τ, providing different b-values according to Equation 1. For b=0, pulses are played every ΔTE as in a conventional CPMG sequence. To keep T2-contrast the same across all b-value images, only the echoes during the spatial-encoding module are acquired. Optionally, the switched encoding gradients can provide additional diffusion-weighting according to Equation 2. Figure 3 shows the achievable b-values as a function of TEeff.
(Eq. 1) $$$b_{built-in}=\frac{1}{12} \gamma^2G_y^2\tau^3$$$
(Eq. 2) $$$b_{switched}= \gamma^2G^2\delta^2(\Delta-\delta/3)$$$
Two petri-dish gel phantoms were prepared. Agarose and nickel-sulfate concentrations were chosen to mimic T1 & T2 of white-matter at 80mT. The parameters were then measured on a 60MHz Bruker-Minispec relaxometer. Sucrose was added to one phantom to model the Apparent Diffusion Coefficient (ADC) of infarcted white-matter (ADC~0.8e-3 mm2/s) [8]. The ADC of the second phantom was close to pure-water (~2e-3 mm2/s). ADC maps were acquired using a diffusion-weighted spin-echo EPI sequence on a clinical 3T scanner. Experimental diffusion-weighted images were then acquired on the portable MRI scanner using a range of b-values (0-245 s/mm2) while holding TEeff constant at 100ms.
Results
Figure 4 shows the petri-dish phantoms along with a short-TE scout image and four
diffusion-weighted images. Nonlinearity
of the built-in encoding field caused some geometric distortion in the 0%-sucrose phantom. As expected, DWI images show greater
signal loss in the 0%-sucrose phantom. Figure
4D shows image intensity versus b-value, providing a good monoexponential
fit to Equation 3. Figure 5 compares ADC maps acquired on the portable scanner and
3T-clinical scanner and summarizes best-fit values for ADC, T1, and
T2 of each gel.
(Eq. 3) $$$s=s_0 exp(-bD)$$$
Discussion
Strong diffusion (ADC) contrast is observed between the two phantoms. The closeness of the measured T2-values for the two gels indicates that the ADC-maps are dominated by diffusion-contrast rather than T2-shinethrough. The ADC-maps for both scanners are generally consistent, though the portable scanner does overestimate ADC in the 0%-sucrose phantom.
We show 2D diffusion-weighted phantom images in a highly-inhomogeneous B0 field as a first step toward using a portable brain scanner for diagnosing brain injury. We also show that dummy refocusing pulses can be used to change the b-value applied by the built-in encoding field without changing TEeff.
In future work, the switched gradient coils will also be used for diffusion-encoding, enabling higher b-values (500-1000 s/mm2). Also, by varying the relative amplitudes of the two gradients, diffusion-encoding will be possible along all three spatial directions. The method will then be extended to 3D acquisitions for testing on humans and eventual application to brain injury.
Acknowledgements
Funding from NIH NIBIB 5T32EB1680, R01EB018976, and K99EB021349. We thank Josh Park, Matt Rosen, Dmitry Novikov, Els Fieremans, and Ona Wu for their helpful suggestions and assistance.References
[1] Wu O, Diffusion MRI (Ch. 31), 2010. [2] Cooley CZ, MRM, 2015. [3] Vaughan T, ISMRM, 2016, p. 498. [4] Casanova F, Single-sided NMR, 2011. [5] Cooley CZ, IEEE Trans Mag, 2017. [6] McDaniel P, ISMRM 2018, p.29. [7] Casabianca L, JMR, 2014. [8] Lavdas I, JMRI, 2013.
Figures



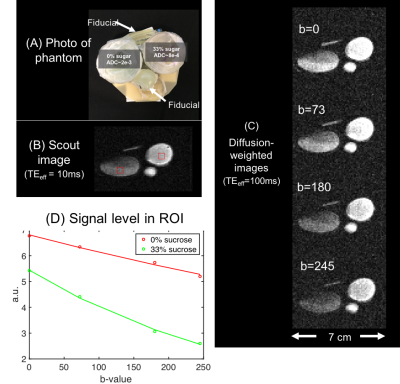
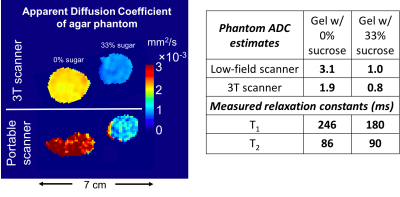
Fig. 5: Left: Comparison of Apparent Diffusion Coefficient (ADC) maps acquired on portable scanner and “ground-truth” maps from a clinical 3T scanner. Both show clear ADC contrast between the two phantoms. Right: Table summarizing average ADC of the two phantoms measured by the two scanners. There is general agreement, though the portable scanner does overestimate the 0%-sucrose ADC. The table also shows measured T1 and T2 values for the gels (target values were 250ms and 90ms for white matter). Because the T2 is almost identical for the two phantoms, the difference in image contrast between the gels must be dominated by diffusion-weighting rather than T2-shinethrough.