1192
Spin-echo imaging at 0.55T using a spiral trajectory1National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Low field MRI combined with modern system engineering is attractive for high quality imaging with reduced production and operation costs. Moreover, there are potential clinical opportunities afforded by the reduced SAR and reduced local susceptibility. However, for a low field system to be clinically useful, neuroimaging must be feasible. Here we explore the potential of spiral neuroimaging at 0.55T to regain SNR that is sacrificed by moving to low field. We show that we can use a 30 ms spiral readout at 0.55T without the need for off-resonance correction and generate a 2.6x gain in SNR compared to Cartesian imaging protocols.
Introduction
With ongoing interest in MRI value, there is renewed attention on low field MRI (<1.5T) due to the reduced system costs [1,2]. New low field MRI systems can employ modern MRI engineering (e.g. shielded gradients, modern magnetic design, local coils and new acquisitions/reconstructions) for improved system performance compared to historical counterparts. Moreover, imaging at low-field may enable new clinical opportunities: improved homogeneity in the static B0 and transmit B1 fields, and reduced SAR, which is important for interventions with metallic devices.
For a low field system to be clinically useful, high signal-to-noise ratio (SNR) and high-resolution neuroimaging must be feasible. Here we sought to develop neuroimaging methods at low field focusing on increased sampling efficiency with non-Cartesian sampling. We show that spiral spin-echo imaging can be used to recover SNR at low field, without the detrimental artifacts typically observed at higher field.
Methods
Study: Data was acquired on a custom 0.55T system (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). Institutional review board approval and informed consent was obtained for human studies.
Spin-echo spiral pulse sequence: A prototype multi-slice spiral-out spin-echo sequence was developed (typical T1-weighted parameters: TR/TE = 450/15 ms, 0.72 x 0.72 mm2 resolution, 5 mm slice thickness, 16 interleaves, acquired at 250 Hz/pixel bandwidth, 10 slices). Total acquisition time was fixed (2 min 24), during which 20 spiral averages or single-average Cartesian acquisitions (at 130 Hz/pixel bandwidth) could be performed with a slice- and parameter- match. Alternatively, SNR-matched images can be generated with only 5 spiral averages (36 s). Transverse and sagittal acquisition were acquired in (n = 4) healthy volunteers.
B0 field mapping: To evaluate field homogeneity at this field strength, B0 field mapping was performed with Cartesian spoiled gradient echo TR/TE = 150/5 & 6 ms, 1.44 x 1.44 mm2 resolution, flip angle 37°, 5 mm slice thickness covering approximately 60 mm in the mid-brain, acquired at 160 Hz/pixel bandwidth in healthy volunteers (n=5).
Reconstruction & Analysis: Image reconstruction and analysis were performed in Matlab (Mathworks, USA). Spiral trajectories were corrected for gradient non-linearities [5] and reconstructed using an iterative CG-SENSE. A pseudo-replica method [6] (100 replicas) was used for SNR estimations [7]. Regions of interest in the white and gray matter were manually drawn in each image.
Results
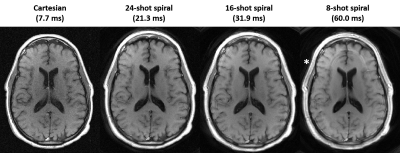
Spiral spin-echo imaging: Comparable T1-weighted contrast was achieved with the spiral spin-echo sequence (Figure 1). No significant artifacts due to blurring were observed and clear SNR improvements are observed compared to Cartesian acquisitions for time-matched protocols.
SNR measurements: Imaging with a longer readout (31.9 ms vs 7.7 ms) per TR is theoretically predicted to yield a 2.0-fold gain in SNR. In vivo, measured white matter SNR increased by 2.6 ± 0.2x (spiral SNR = 49.7 ± 8.7 vs. Cartesian SNR = 19.4 ± 3.3) and gray matter SNR increased by 2.6 ± 0.5x (spiral SNR = 42.8 ± 10.3 vs. Cartesian SNR = 16.5 ± 2.8) for the time-matched protocols (Figure 2).
B0 map inhomogeneity: The brain B0 maps at 0.55T are displayed in Figure 3. Mean off-resonance over the whole head was measured to be -4.1 ± 28.2 Hz. Such field homogeneity allowed for spiral readout lengths up to 30 ms without significant blurring artefacts (Figure 4).
Discussion and conclusions
Low field MRI may potentially reduce the production and operating costs for clinical imaging, though maintaining diagnostic SNR is vital. This research demonstrates that baseline SNR from standard Cartesian acquisitions can be improved upon using long spiral readouts, which can take advantages of the longer tissue T2* and reduced B0 field inhomogeneity at low field. Alternatively, the more efficient readout strategy can be used to reduce imaging time for equivalent SNR (Figure 2). A long readout will limit the number of interleaved slices per TR, though this may be mitigated by the acquisition efficiency of spirals. Future work will explore the optimization of image contrast for diagnostic spiral neuroimaging at low field.Acknowledgements
This work was supported by the NHLBI DIR (Z01-HL006039, Z01-HL005062, Z1A-HL006213, Z1A-HL006214).References
[1] Y. Zhuang, et al., Validation of diffusion measurements obtained on a 0.35T MR in Malawi: Important insights for radiologists in low income settings with low field MRI. Magn Reson Imaging, 2018. 45: p. 120-128.
[2] O.P. Simonetti, R. Ahmad. Low Field Cardiac MRI: A Compelling Case for CMR’s Future. Circ Cardiovasc Imaging. 2017; 10(6): . doi:10.1161.
[3] Z. Li, et al. A Spiral Spin-Echo MR Imaging Technique for Improved Flow Artifact Suppression in T1-Weighted Postcontrast Brain Imaging: A Comparison with Cartesian Turbo Spin-Echo. American Journal of Neuroradiology. 2016; 37 (4) 642-647.
[4] G. Capture, et al. A medical device-grade T1 and ECV phantom for global T1 mapping quality assurance-the T1 Mapping and ECV Standardization in cardiovascular magnetic resonance (T1MES) program. J Cardiovasc Magn Reson. 2016;18(1):58.
[5] A.E. Campbell, et al. Real-time distortion correction of spiral and echo planar images using the gradient system impulse response function. Magn Reson Med. 2016;75(6):2278-85. doi: 10.1002/mrm.25788.
[6] P.M. Robson, et al. Comprehensive Quantification of Signal-to-Noise Ratio and g-Factor for Image-Based and k-Space-Based Parallel Imaging Reconstructions. Magn Reson Med. 2008; 60(4): 895–907.
Figures