1182
Rapid, Time-Resolved Brain Imaging with Multiple Clinical Contrasts using Wave-Shuffling.1Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Department of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Previously, we presented Wave-Shuffling: a technique that combines random-sampling and the temporal low-rank priors of T2-shuffling with the sinusoidal gradients of Wave-CAIPI to achieve highly-accelerated, time-resolved acquisitions. In this work, we optimize and apply Wave-Shuffling to T2-SPACE and MPRAGE to achieve rapid, 1 mm-isotropic resolution, time-resolved imaging of the brain where we recover a time-series of clinical contrast. We also present preliminary explorations into Joint-Contrast Wave-Shuffling to boost signal-to-noise ratio and reconstruction-conditioning at high accelerations.
Introduction
Previously, we presented Wave-Shuffling: a technique that combines random-sampling and the temporal low-rank priors of $$$T_2-$$$shuffling1 with the sinusoidal gradients of Wave-CAIPI2 to achieve highly-accelerated, time-resolved acquisitions. In this work, we optimize and apply Wave-Shuffling to $$$T_2-$$$SPACE and MPRAGE to achieve rapid, $$$1 mm-$$$isotropic resolution, time-resolved imaging of the brain where we recover a time-series of clinical contrast. We also present preliminary explorations into Joint-Contrast Wave-Shuffling to boost signal-to-noise ratio and reconstruction-conditioning at high accelerations.
Wave-Shuffling Model And Reconstruction
Forward Model. A succinct description of Wave-Shuffling is as follows:$$b=\underbrace{MF_{yz}WF_{x}RE \Phi}_{\triangleq A}\;c,$$where $$$c$$$ represents the underlying temporal coefficients from the Shuffling model, $$$\Phi$$$ is the temporal basis that expands the coefficients to a model-based time-series of images, $$$E$$$ is the ESPIRiT3 operator, $$$R$$$ is the readout $$$(x)$$$ resize operator to a larger FOV to account for the readout alias-spreading as a function of spatial phase-encode positions $$$(y, z)$$$ induced by Wave-CAIPI, $$$F$$$ is the Fourier Transform, $$$W$$$ is the Wave-CAIPI Point Spread Function (PSF), $$$M$$$ is the phase-encode sampling mask, and $$$b$$$ is the acquired data.
Reconstruction. The reconstruction problem is posed as follows:$$c^*=\underset{c}{\mathrm{argmin}}\;\frac{1}{2}||Ac-b||_2^2+\lambda||T(c)||_1,$$where $$$T$$$ is the spatial Wavelet transform and $$$\lambda$$$ is the regularization. This problem is solved with BART4 using FISTA5.
Methods
A healthy subject was scanned on a 3T scanner using 32-channel head coil with$$$\;T_2-$$$SPACE and MPRAGE sequences that were modified to play sinusoidal gradients during readout and randomly reorder phase-encode sampling. The matrix size for both cases were $$$256\times256\times256$$$ at $$$1mm$$$ isotropic.
MPRAGE. Fully-sampled (randomly ordered) data with and without sinusoidal readout gradients was acquired using $$$TI=1.2s,TR=2.5s,$$$ flip angle of $$$8^\circ,T_{\text{acquisition}}=11\;\text{minutes}$$$ and an echo-train length of $$$256$$$. The data was retrospectively under sampled to contain $$$100\%,50\%,25\%$$$ of the possible $$$256\times256$$$ phase-encode points, which we denote $$$R1,R2,R4$$$ respectively.
$$$T_2-$$$SPACE. Fully-sampled (randomly ordered) data with $$$89\%\;$$$Partial Fourier (PF) in the$$$\;y\;$$$direction, was acquired with a variable flip angle train6 of echo-train length of$$$\;196$$$, echo spacing of$$$\;4.6ms$$$.$$$TR=3.2s,T_{\text{acquisition}}=16\;\text{minutes}$$$ and sinusoidal gradients. The data was retrospectively under sampled to contain $$$25\%$$$ of the possible $$$228\times256\;$$$phase-encode points ($$$22\%$$$ of $$$256\times256$$$), which we denote $$$R4$$$. PF was accounted for by applying homodyne projection-onto-convex-sets (POCS) to the reconstructed coefficients.
Wave Parameter Optimization. Through simulations, it was observed that larger readout alias-spreading provides better reconstruction conditioning. This spreading is proportional to the maximum amplitude of the sinusoidal gradient, which is maximized by minimizing the number of cycles given gradient-slew and readout duration limitations. In our previous work7, we showed that small cycles create large corkscrew k-space trajectories that result in signal mixing across partition-encoding due to $$$T_1/T_2\;$$$recovery over the acquisition-train, which causes ringing artifacts in standard wave-CAIPI. The Shuffling model resolves signal-recovery over time, allowing for small cycles without artifacts. Consequently, for$$$\;T_2-$$$SPACE, we used$$$\;2\;$$$ cycles with a gradient maximum-amplitude of $$$25mT/m$$$. For MPRAGE with a lower readout bandwidth,$$$\;8\;$$$cycles with a gradient maximum-amplitude of $$$16mT/m$$$ was used for good conditioning while avoiding artifacts of lower-cycle cases with undesirable velocity-encoding.
Results
Figure 1 shows that Shuffling without Wave-CAIPI provides clean reconstruction at $$$R1$$$ with increased noise at $$$R2$$$ and artifacts at $$$R4$$$. Wave-Shuffling achieves clean results at $$$R4$$$ and more closely resembles Shuffling $$$R1$$$ upto noise considerations. Figure $$$2$$$ demonstrates Wave-Shuffling's ability to recover a sharp image of the clinical $$$T_2-$$$SPACE contrast at $$$R4$$$, which is the virtual echo at the center of the echo-train (top-panel), as well as the time-series of signal evolution (bottom-panel). Figure $$$3$$$ is an animated GIF that shows the signal-evolution from Wave-Shuffling of $$$R4$$$ MPRAGE and $$$R4\;T_2-$$$SPACE.Joint Contrast Considerations
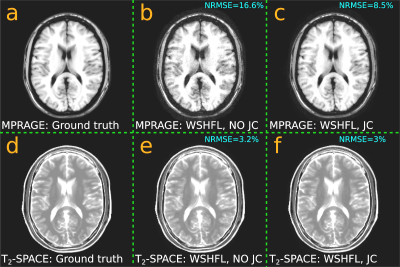
Given recent developments of joint reconstruction for multi-contrast acquisitions8-11, we present our preliminary explorations of Joint-Contrast Wave-Shuffling. The magnitude coefficients of the above reconstructed $$$R4$$$ MPRAGE and $$$R4\;T_2$$$-SPACE data are registered with respect to each other using FSL12 (since the same subject was scanned on different sessions), and a locally low-rank threshold is applied to promote structural similarities. The result is depicted in Figure 4, where the prior is seen to help remove visual noise with a marginal improvement to normalized root mean squared error with respect to the respective $$$R1$$$ magnitude coefficients.
We plan to explore this direction further by incorporating the different signal evolution of MPRAGE and $$$T_2-$$$SPACE into $$$\Phi$$$ so as to enforce joint-contrast with the data-consistency term. Simulation results of the same are presented in Figure 5. Brain web13 phantom courtesy of Dr. Bo Zhao.
Conclusion
We successfully optimized and applied Wave-Shuffling to $$$T_2-$$$SPACE and MPRAGE to provide fast, time-resolved imaging with clinical contrasts at $$$3T$$$. Preliminary results using Joint-Contrast was also presented, which helps de-noise reconstruction at high accelerations.Acknowledgements
This work was supported in part by NIH research grants: R01MH116173, R01EB020613,R01EB019437, U01EB025162, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043.
References
- Tamir, J.I., Uecker, M., Chen, W., Lai, P., Alley, M.T., Vasanawala, S.S. and Lustig, M., 2017. T2 shuffling: Sharp, multicontrast, volumetric fast spin‐echo imaging. Magnetic resonance in medicine, 77(1), pp.180-195.
- Bilgic, B., Gagoski, B.A., Cauley, S.F., Fan, A.P., Polimeni, J.R., Grant, P.E., Wald, L.L. and Setsompop, K., 2015. Wave‐CAIPI for highly accelerated 3D imaging. Magnetic resonance in medicine, 73(6), pp.2152-2162.
- Uecker, M., Lai, P., Murphy, M.J., Virtue, P., Elad, M., Pauly, J.M., Vasanawala, S.S. and Lustig, M., 2014. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magnetic resonance in medicine, 71(3), pp.990-1001.
- Uecker, M., 2018. Mrirecon/Bart. Latest version located at https://github.com/mrirecon/bart.
- Beck, A. and Teboulle, M., 2009. A fast iterative shrinkage-thresholding algorithm for linear inverse problems. SIAM journal on imaging sciences, 2(1), pp.183-202.
- Mugler, J.P., Kiefer, B. and Brookeman, J.R., 2000, April. Three-dimensional T2-weighted imaging of the brain using very long spin-echo trains. In Proceedings of the 8th Annual Meeting of ISMRM (p. 687).
- Polak, D., Setsompop, K., Cauley, S., Gagoski,B., Batt, H., Maier. F., Wald, L., and Bilgic, B., 2017. Wave-CAIPI for Highly Accelerated MP-RAGE Imaging. International Society for Magnetic Resonance in Medicine, 25th Scientific Meeting, Hawaii, USA.
- Bilgic, B., Kim, T.H., Liao, C., Manhard, M.K., Wald, L.L., Haldar, J.P. and Setsompop, K., 2018. Improving parallel imaging by jointly reconstructing multi‐contrast data. Magnetic resonance in medicine, 80(2), pp.619-632.
- Kilic, T., Ilicak, E., Çukur, T. and Saritas, E., 2017. Improved SPIRiT Operator for Joint Reconstruction of Multiple T2-Weighted Images. International Society for Magnetic Resonance in Medicine, 25th Scientific Meeting, Hawaii, USA, p. 5165.
- Bilgic, B., Cauley, S.F., Wald, L.L. and Setsompop, K., 2018. Joint SENSE Reconstruction for Faster Multi-Contrast Wave Encoding. International Society for Magnetic Resonance in Medicine 27th Scientific Meeting, Paris, France, p. 940.
- Tamir, J., Taviani, V., Vasanawala, S. and Lustig, M., 2017. T1-T2 Shuffling: Multi-Contrast 3D Fast Spin-Echo with T1 and T2 Sensitivity. International Society for Magnetic Resonance in Medicine, 25th Scientific Meeting, Hawaii, USA.
- Jenkinson, M., Beckmann, C.F., Behrens, T.E., Woolrich, M.W. and Smith, S.M., 2012. Fsl. Neuroimage, 62(2), pp.782-790.
- Collins, D., Zijdenbos, A., Kollokian, V., Sled, J., Kabani, N., Holmes, C. and Evans, A., 1998. Design and construction of a realistic digital brain phantom. IEEE Trans. Med. Imaging, vol. 17, pp. 463–468.
Figures