1179
Multi-Point 5D Flow MRI - Accelerated Cardiac- and Respiratory-Motion Resolved Mapping of Mean and Turbulent Velocities in 4 Minutes1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Synopsis
This work presents a framework for respiratory motion-resolved multi-point 4D Flow MRI (MP 5D Flow MRI) of mean and turbulent velocities in 4 minutes using a combination of Cartesian Golden angle undersampling, data-driven motion detection and locally low-rank imaging reconstruction. In an imaging study with 9 volunteers, 7-point 5D Flow MRI was compared to a standard, navigator-gated 4-point 4D Flow MRI parallel imaging protocol. Results demonstrate that flow fields from MP 5D Flow MRI in end-expiration agree well with 4D Flow MRI data while scan time was reduced by a factor of 4.5. In addition, MP 5D Flow MRI provides higher accuracy for low velocities and allows assessing TKE over a larger dynamic range compared to 4D Flow MRI.
Introduction
Respiratory-motion resolved 4D flow MRI1–3 acquires data independently of respiratory motion and exploits similarities among respiratory motion states in the reconstruction. This work presents a framework which combines respiratory-motion resolved reconstruction with multipoint encoding (MP 5D Flow MRI) to provide a robust protocol for the assessment of velocity maps and turbulent kinetic energy4 as well as flow conditions in different respiratory motion states.
Methods
Data Acquisition and Reconstruction
Data acquisition and data-driven motion state detection was performed using an undersampled tiny Cartesian Golden angle approach as illustrated in Figure 15. Given the observation that the low-rank property of 4D flow MRI data varies as a function of spatial location6, a locally low-rank (LLR)7,8 approach was used to exploit correlations among heart phases (hp) and respiratory motion states (ms). Data for each velocity encoding were reconstructed separately as
$$\hat{X}_{k_v}=arg \min_x ||\Omega \mathcal{FS}(X_{k_v})-y||_2^2+\lambda\sum_b||R_b(X_{k_v})||_*$$
with undersampling operator $$$\Omega$$$, Fourier transform $$$\mathcal{F}$$$, coil sensitivities $$$\mathcal{S}$$$, k-space data $$$y$$$ and regularization weight $$$\lambda$$$. As illustrated in Figure 2a, the operator $$$R_b$$$ selects patches of size $$$n_x \times n_y \times n_z$$$ in the image from all $$$N_{hp}$$$ heart phases and $$$N_{ms}$$$ motion states and transforms them into a Casorati matrix with dimensions $$$n_x n_y n_z \times N_{hp} N_{ms}$$$.
To ensure accurate quantification of mean and turbulent velocities, a multi-point encoding scheme 9 with 7 different velocity encodings was used 2x3 kv points + 1 kv = 0 reference) and a Bayesian approach was employed to combine the data. As illustrated in Figure 2b, velocities and intra-voxel dephasing are determined by maximizing their posterior probability given the measured data9.
Imaging Study
Flow in the aortic arch of 9 healthy volunteers was acquired on a 3T Philips Ingenia system (Philips Healthcare, Best, the Netherlands) using the proposed MP 5D Flow MRI and a standard, navigator-gated 4-point 4D Flow MRI approach 10 with a spatial resolution of $$$2.5\times2.5\times2.5mm^3$$$ and 25 cardiac phases. Using the proposed method, velocities were encoded with $$$50cm/s$$$ and $$$150cm/s$$$$ and scan time was fixed to 4 minutes. The 4D reference data (venc: 150cm/s) were acquired using 5mm pencil-beam navigator gating on the diaphragm and, accordingly, the effective scan time depended on the breathing pattern of the subjects. 5D data were sorted into four respiratory motion states. Equation 1 was solved using BART11. LLR reconstructions using only data in end-expiration (EXP) and without gating (NG) were compared to reconstructions exploiting respiratory motion states (5D). Moreover, 5D results obtained with LLR were compared to XD-GRASP5. Hyperparameters for LLR and XD-GRASP were automatically tuned for best agreement of velocities in the aorta relative to the 4D reference data.
Data Analysis
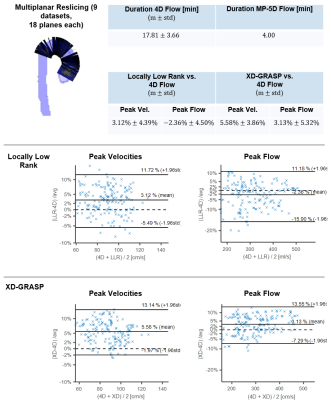
Multi-planar reslicing along the aorta was performed using VMTK (www.vmtk.org) and peak flow and peak through-plane velocities were compared using Bland-Altman analysis12. TKE maps were calculated as in 13.
Results
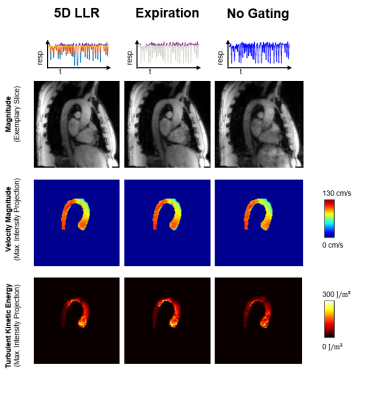
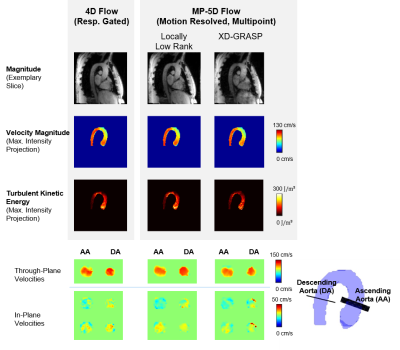
Figure 3 compares reconstruction results of 5D LLR, EXP and NG reconstructions. Magnitude images and TKE maps from 5D LLR reconstructions show less artifacts compared to EXP and NG. Exemplary results of magnitude and velocity magnitude images in expiration obtained with 5D LLR and XD-GRASP are compared relative to the 4D reference results in Figure 4. Maximum intensity projections of velocity magnitudes are similar for all methods whereas TKE maps and magnitude images show more residual aliasing noise for XD-GRASP when compared to 5D LLR. Figure 5 compares results obtained for all 9 volunteers using Bland-Altman analysis. Scan durations for 4D were 17.81+/-3.66min compared to 4 minutes for 5D LLR. On average 5D LLR and XD-GRASP both show good agreement of peak velocities and peak flow relative to the 4D reference.Discussion
Respiratory-motion resolved reconstruction improves
reconstruction accuracy relative to using data from end-expiration only. The
acquisition time of MP 5D Flow MRI was 4.5 times shorter on average compared to
the 4D Flow MRI reference. Comparing 5D LLR reconstruction with XD-GRASP, both
methods showed good agreement of velocity data, however, TKE maps could be more faithfully reconstructed using the 5D LLR
approach.Conclusion
Respiratory motion-resolved multi-point 5D Flow MRI allows for breathing-pattern independent mapping of mean and turbulent velocities in 4 minutes.Acknowledgements
No acknowledgement found.References
1. Cheng JY, Zhang T, Alley MT, Uecker M, Lustig M, Pauly JM, Vasanawala SS. Comprehensive Multi-Dimensional MRI for the Simultaneous Assessment of Cardiopulmonary Anatomy and Physiology. Scientific Reports. 2017;7(1):6–9.
2. Jonas Walheim and Sebastian Kozerke. 5D Flow MRI – Respiratory Motion Resolved Accelerated 4D Flow Imaging Using Low-Rank + Sparse Reconstruction. In: Proceedings of the 26th Annual Meeting of ISMRM. Presented at the ISMRM. 2018. p. 0032.
3. Bastkowski R, Weiss K, Maintz D, Giese D. Self-gated golden-angle spiral 4D flow MRI. Magnetic Resonance in Medicine. 2018;80(3):904–913.
4. Dyverfeldt P, Sigfridsson A, Kvitting JE, Ebbers T. Quantification of intravoxel velocity standard deviation and turbulence intensity by generalizing phase-contrast MRI. 2006;(56):850–858.
5. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magnetic resonance in medicine. 2016;75(2):775–788.
6. Giese D, Schaeffter T, Kozerke S. Highly undersampled phase-contrast flow measurements using compartment-based k-t principal component analysis. Magnetic Resonance in Medicine. 2013.
7. Zhang T, Pauly JM, Levesque IR. Accelerating parameter mapping with a locally low rank constraint. Magnetic Resonance in Medicine. 2015;73(2):655–661.
8. Trzasko J, Manduca A, Borisch E. Local versus global low-rank promotion in dynamic MRI series reconstruction. In: Proc. Int. Symp. Magn. Reson. Med. 2011. p. 4371.
9. Binter C, Knobloch V, Manka R, Sigfridsson A, Kozerke S. Bayesian multipoint velocity encoding for concurrent flow and turbulence mapping. Magnetic Resonance in Medicine. 2013;69(5):1337–1345.
10. Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll C-J, Ebbers T, Francios CJ, Frydrychowicz A, Geiger J, Giese D, et al. 4D flow cardiovascular magnetic resonance consensus statement. Journal of Cardiovascular Magnetic Resonance. 2015;17(1):1.
11. Ong F, Uecker M, Tariq U, Hsiao A, Alley MT, Vasanawala SS, Lustig M. Berkeley advanced reconstruction toolbox. Magnetic Resonance in Medicine. 2015;73(2):828–842.
12. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. The statistician. 1983:307–317.
13. Dyverfeldt P, Sigfridsson A, Kvitting JPE, Ebbers T. Quantification of intravoxel velocity standard deviation and turbulence intensity by generalizing phase-contrast MRI. Magnetic Resonance in Medicine. 2006;56(4):850–858.
14. Cheng JY, Hanneman K, Zhang T, Alley MT, Lai P, Tamir JI, Uecker M, Pauly JM, Lustig M, Vasanawala SS. Comprehensive motion-compensated highly accelerated 4D flow MRI with ferumoxytol enhancement for pediatric congenital heart disease. Journal of Magnetic Resonance Imaging. 2016;43(6):1355–1368.
15. Zhang T, Cheng JY, Chen Y, Nishimura DG, Pauly JM, Vasanawala SS. Robust self-navigated body MRI using dense coil arrays. Magnetic Resonance in Medicine. 2016;76(1):197–205.
Figures