1172
Geometric Distortions and Signal-To-Noise Ratio of Conventional and Inner Fields-of-View for T2*-Weighted Echo-Planar Imaging of the Spinal Cord1Department of Systems Neuroscience, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Synopsis
Geometric distortions and signal-to-noise ratios (SNR) of T2*-weighted echo-planar imaging (EPI) of the spinal cord are compared for conventional and inner-FOV acquisitions based on 2D-selective RF (2DRF) excitations. For conventional acquisitions, the required FOV increases with the in-plane object size yielding more pronounced distortions, prolonged echo times (TEs), and reduced SNR. For inner-FOV acquisitions, the FOV is small and independent of the object size yielding only minor distortions. The 2DRF pulse duration must be adapted for larger object sizes resulting in slightly prolonged TEs but overall TEs remain shorter and SNR values are larger than for conventional acquisitions.
Introduction
Echo-planar imaging (EPI)1 of inner-fields-of-view (FOV) based on spatially Two-dimensional selective RF (2DRF) excitations2,3 allows to focus the FOV to an inner target region without aliasing4 which can reduce geometric distortions and the echo time (TE) and, thus, increase the image quality and signal-to-noise ratio (SNR). It has been applied successfully for diffusion-weighted imaging of the spinal cord,5,6 and T2*-weighted imaging of small brain regions,7 however, its potential for blood-oxygenation-level-dependent (BOLD) functional neuroimaging of the spinal cord8 is still under research. In this study, conventional and 2DRF-based inner-FOV T2*-weighted EPI acquisitions are compared and evaluated concerning geometric distortions and SNR for different in-plane object sizes.Methods
The basic EPI pulse sequences used for conventional slice-selective and inner-FOV acquisitions based on 2DRF excitations are shown in Fig. 1.
The geometric setups for acquisitions of inner targets like the spinal cord are sketched in Fig. 2. For conventional acquisitions, the required phase-encoding FOV depends on the in-plane body or object size in the phase encoding direction. Some aliasing may be tolerable as long as parallel acquisition techniques (PAT) are not used and the desired target region is not affected (Fig. 2a). For inner-FOV acquisitions, the FOV only has to cover the target region, i.e. is independent of the object size, but the field-of-excitation (FOE) of the 2DRF pulse must be large enough to position the side excitations outside of the object to avoid unwanted signal contributions. (Fig. 2b) This means that the FOE and, thus, the 2DRF pulse length depends on the in-plane object size.
Measurements were performed on a 3T whole-body MR-system (PrismaFit, Siemens Healthineers) using a 20-channel head coil for the phantom and the neck coil elements of a 64-channel head-neck coil for in vivo acquisitions. Healthy volunteers were investigated after their informed consent was obtained. All acquisitions were performed using an in-plane resolution of 1.0×1.0mm2 and a slice thickness of 5mm with a fixed repetition time (TR) of 4030ms (in vivo) and 3000ms (phantom). Protocols for a target size (inner-FOV) of 32mm and in-plane object sizes between 160mm (cervical spinal cord) and 416mm (thoracic/lumbar spinal cord in obese patients) were considered yielding FOVs between 96mm and 224mm for conventional acquisitions and FOEs between 110mm and 250mm for inner-FOV acquisitions (2DRF pulse durations between 11.5ms and 26.0ms). Conventional acquisitions were performed without and with PAT because the receive coils, which are available for the spinal cord may not be feasible for PAT in lower cord sections. Thus, conventional EPI was performed with TEs between 23ms and 46ms (PAT) and, 41ms and 86ms (no PAT). Inner-FOV EPI was performed with 40% phase-encoding oversampling to consider imperfections of the 2DRF excitation profile (resolution 2.5×10.0mm2) yielding TEs between 26ms and 33ms.
All in vivo tests (12 slices) were performed in the cervical spinal cord for a direct comparison of distortions and SNR for different object sizes.
SNR values in the spinal cord were determined using a self-written IDL procedure by taking the voxel-wise ratio of the mean and standard deviation of 20 measurements and averaging this ratio over the spinal cord cross-section in each slice.
Result and Discussion
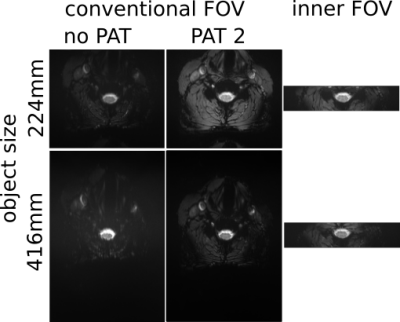
The images in Fig. 3 demonstrate that the geometric distortions are more pronounced in conventional than in inner-FOV acquisitions and increase with the object size due to the larger FOV required. In vivo images of a healthy volunteer are shown in Fig. 4. As in the phantom acquisitions, increased distortions are visible for conventional acquisitions together with a significant SNR reduction for the large object size due to the much longer TE required.
Figure 5 presents the SNR values in a single slice and averaged over 10 slices as a function of the object size. Inner-FOV acquisitions show a good SNR with only a slight decrease with the object size due to an increased FOE and a correspondingly prolonged 2DRF pulse and TE. Conventional acquisitions without PAT have a lower SNR and show a stronger decrease with the object size due to the increased FOV and TE which are longer than inner FOV acquisitions. With PAT, conventional acquisitions show an improved SNR for large object sizes due to the shorter echo train and TE but still have a lower SNR compared to inner-FOV acquisitions. For smaller object sizes, PAT acquisitions show a bad performance because aliasing artifacts interfere with PAT.
Conclusion
In conclusion, inner-FOV acquisitions provide reduced geometric distortions and an improved SNR for T2*-weighted imaging of the spinal cord, in particular for lower cord sections and obese patients, and could help to enhance BOLD-based neuroimaging.Acknowledgements
No acknowledgement found.References
1. Mansfield P. Multi-planar image formation using NMR spin echos. J Phys C. 1977; 10: 55-58
2. Bottomley PA, Hardy CJ. Two-dimensional spatially selective spin inversion and spin-echo refocusing with a single nuclear magnetic resonance pulse. J Appl Phys. 1987; 62: 4284-4290.
3. Pauly J, Nishimura D, Macovski A. A k-space analysis of small-tip-angle excitations. J Magn Reson. 1989; 81: 43-56.
4. Rieseberg S, Frahm J, Finsterbusch J, Two-dimensional spatially-selective RF excitation Pulses in echo-planar imaging. Magn Reson Med. 2002; 47: 1186-1193
5. Saritas EU, Cunningham CH, Lee JH, Han ET, Nishimura DG. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med. 2008; 60: 468-473.
6. Finsterbusch J. High-resolution diffusion tensor imaging with inner field-of-view EPI. J Magn Reson Imaging. 2009; 29: 987-993
7. Finsterbusch J. Functional neuroimaging of inner fields-of-view with 2D-selective RF excitations. Magn Reson Imaging. 2013; 31: 1228–1235
8. Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct Evidence for Spinal Cord Involvement in Placebo Analgesia. Science. 2009; 326: 404
9. Oelhafen M, Pruessmann KP, Kozerke S, Boesiger P. Calibration of Echo-Planar 2D-selective RF excitation pulses. Magn Reson Med. 2004; 52: 1136-1145
Figures