1060
Parallel Transmit Optimized Spiral Composite Adiabatic Pulses for 3D Spatial-Spectral Selectivity in Spectroscopy1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
In this abstract we demonstrated by simulation and phantom experiments the feasibility of designing composite refocusing pulse for spectroscopy using parallel transmission, featuring inherent 2D spatial and spectral selectivity along with improved immunity to B0 non-uniformity and B1+ heterogeneity while simultaneously providing lipid and water suppression and short echo times of ~18ms.
Introduction
For magnetic resonance spectroscopy, the ability to observe and quantify metabolites of interest is usually compromised by B0 and B1+ inhomogeneity, compromised echo times and SNR for improved spatial selectivity and the necessity to accommodate background signal suppression (i.e. water and lipids) depending on the application. Typical UHF spin echo spectroscopy sequence uses pairs of adiabatic refocusing pulses for high bandwidth spatial localization, along with additional pulses for water and fat suppression. However, this comes at the cost of a long echo times high RF power deposition along with limited flexibility to perform a variety of studies in the brain and body such as relaxometry. In this study, we present a novel composite pulse that uses an adiabatic envelope and parallel transmission (pTx) optimized subpulses that have inherent 2D-spatial and 1D-spectral selectivity. As a proof of concept, we showed an example of designing a composite-spiral refocusing pulse with both 2D-spatial and 1D-spectral selectivity with an intended application to prostate spectroscopy. Both simulation and phantom validations were conducted to evaluate the feasibility and performance.Methods
- B0/B1+ Field Mapping
For the pTx subpulse design, the B0 and B1+ fields of a prostate spectroscopy phantom were acquired on a Siemens Magnetom 7T scanner (German, Erlangen, Siemens) using an 8-channel transmit and 32-channel receive coil. The per-channel B1+ mapping was calculated using the previously described hybrid method that combines relative B1+ mappings with AFI measurement (Ref 1). The B0 mapping was acquired by a dual-echo GRE sequence. (Figure 1)
- Composite Pulse Design
The pTx sub-pulse was first designed using the spatial domain method (Ref 2), incorporating a mask of the prostate phantom along with the acquired B0 and B1+ field maps. A spiral-out excitation k-space trajectory with a balanced gradient design was used to achieve the desired excitation pattern (Figure 2). The composite pulse was then synthesized by modulating the subpulse with an adiabatic pulse envelope (Ref3). The duration of the sub-pulse, denoted as tp, dictates the periodicity of frequency response of the adiabatic envelope after discretization. For a composite pulse consisting of N subpulses, the time bandwidth product has a relationship of R = BW * N * tp. For prostate spectroscopy, the metabolites of interest range from 3.2ppm (choline) to 2.6ppm (citrate), requiring at least 0.6ppm of passband in the composite pulse. To suppress water (4.65 ppm) and lipid (1.3ppm) at stopband, the subpulse was tailored to have a duration of 1ms, yielding a 1kHz periodicity in the frequency response of the composite pulse when imaging at 7T. To satisfy the passband requirement, an 18-point HS1R9 adiabatic pulse was used as the envelope pulse and gave the composite pulse an approximate bandwidth of 500Hz.
- Phantom Validation of Selectivity
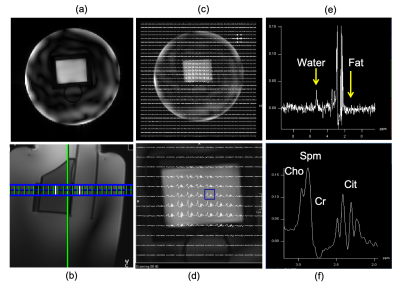
To investigate the robustness of the designed composite pulse against off-resonance, the spatial and spectral profile were calculated for off-resonance ranging from -1,000 Hz to 1,000 Hz in a step size of 10 Hz. The crushed spin echo profile was also calculated to estimate the spatial and spectral profile after spoiling the signals with imperfect refocusing (Figure 3). Customized 3D GRE sequence and spectroscopy sequence were implemented to read the designed pulse and verify the designed spatial and spectral profile. For the spectroscopy, a slice selective excitation pulse was used, yielding a 3D spatial excitation when combined with the designed 2D spatially selective refocusing pulse.
Results
Both the simulation and phantom experiment showed that the spatial and spectral profiles of the designed composite pulse closely matched the desired pattern (figure). The simulated crushed spin echo profile had an approximately 260Hz of 95% passband (Figure 3), with stopband for water and fat approximately 320 Hz. Inherent water and fat suppression (Figure 4) of the designed pulse were confirmed in the phantom experiment.Discussion
To our best knowledge, this is the first demonstration of designing spectral and spatial selective pulse using parallel transmission, with an example of composite refocusing pulse for prostate spectroscopy. This novel design approach enabled field following excitation and extended selectivity to the spectral domain. As a preliminary result, there is still great potential for improvement such as by gradient optimization and adiabatic pulse design. The proposed method could also be applied to design the excitation pulse which will enable even more flexibility and potentially improved performance for other useful applications in the brain and body.Conclusion
We have demonstrated the feasibility of designing a spatial and spectral selective composite refocusing pulse using pTx optimized spiral sub-pulses, with an intended application to prostate spectroscopy. The tailored composite adapts to the measured B0 and B1+ fields, yielding improved robustness against field non-uniformities, along with potentially great flexibility in different applications.Acknowledgements
Supported by: NCI R01 CA155268, NIBIB P41 EB015894.References
Wu, X., Auerbach, E. J., Vu, A. T., Moeller, S., Van de Moortele, P. F., Yacoub, E., & Uğurbil, K. (2019). Human Connectome Project-style resting-state functional MRI at 7 Tesla using radiofrequency parallel transmission. NeuroImage, 184, 396-408.
Grissom, W., Yip, C. Y., Zhang, Z., Stenger, V. A., Fessler, J. A., & Noll, D. C. (2006). Spatial domain method for the design of RF pulses in multicoil parallel excitation. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 56(3), 620-629.
Garwood, M., & Uğurbil, K. (2018). RF pulse methods for use with surface coils: Frequency-modulated pulses and parallel transmission. Journal of Magnetic Resonance, 291, 84-93.
Figures