1044
T2 Weighted Whole-Brain Intracranial Vessel Wall Imaging at 3 Tesla with Cerebrospinal Fluid suppression1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Siemens Healthcare Pte. Ltd, Singapore, Singapore, Singapore, 3Department of Neurology, Shenzhen No.2 People’s Hospital, Shenzhen, China
Synopsis
T2 weighting intracranial vessel wall MR imaging provides good contrast to discriminate various important plaque components and is a good tool to differentiate intracranial vasculopathy. However, the strong CSF signal in T2-weighted images interfere the depiction of the vessel wall. In this study, we proposed to use T2IR preparation module combined with T2-weighted SPACE for whole brain intracranial vessel wall imaging, the T2IR pulses were used to suppress CSF while minimizing its effect on the signal reduction from T2w SPACE. This new technique was first evaluated in healthy volunteers and then tested on some stroke patients.
INTRODUCTION
T1-weighted intracranial vessel wall MR imaging has gain popularity due to its intrinsic ability to suppress cerebrospinal fluid (CSF) signal and assess contrast enhancement of the intracranial plaques (1). Recent studies reported that T2-weighted intracranial magnetic resonance vessel wall imaging is a complementary tool to differentiate intracranial vasculopathy (2), and T2-hyperintensity foci were more frequently observed within plaques of symptomatic stenosis than within the plaques of asymptomatic stenosis (3). However, the strong CSF signal in T2-weighted images interferes with the depiction of the vessel wall and thus dark CSF is required (4). Inversion pulse has been used to suppress CSF in T2-weighted TSE images (5), but the resulting imaging suffered from significant signal-to-noise ratio (SNR) deficiency, which was compromised with low spatial resolution (0.8mm3 iso) at ultra-high field system (7 Tesla). A novel technique named T2-FLAIR (hereafter T2IR) has been proposed to suppress CSF with improved SNR (6). In this work, we combined the T2IR preparation module with T2-weighted SPACE to achieve whole brain intracranial artery wall imaging at 3 Tesla.METHEODS
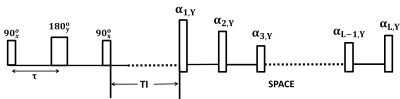
Sequence design: The timing diagram of the proposed sequence (refer to as T2IR SPACE) was shown in Figure 1. The preparation pulse, refer to as T2IR, tips the magnetization down to –z axis after T2 preparation (6). It consists of three non-selective RF pulses $$$90_x^o-\tau-180_y^o-\tau-90_x^o$$$, the first two pulses $$$90_x^o-180_y^o$$$ act like spin echo and the spins experience T2 decay during 2τ, the last $$$90_x^o$$$ pulse inverts the transverse magnetization formed by the spin echo to the negative longitudinal axis. Upon the inversion time (TI) selected to null CSF, SPACE sequence is executed for image acquisition. In-vivo Experiments: All experiments were performed on a 3T MR system (TIM TRIO, Siemens, Erlangen) equipped with 32 channel head coil. Six healthy volunteers (3 females; age 21-61 years old) were recruited for this study. Four patients (1 female, 33-52 years old) who were diagnosed with intracranial arterial stenosis were recruited. All studies were approved by IRB. Informed consents were obtained from all participants. For each volunteer, the imaging volume of interest was first localized. Intracranial artery wall imaging with whole brain coverage (7,8) was performed using T2IR-SPACE, IR-SPACE and T2 SPACE in a random order. The common imaging parameters for these three sequences were: FOV=170×170mm2, matrix size= 288×288, spatial resolution=0.6mm3 iso, iPAT2, scan time=11min40s; specific parameters for each sequence were: T2 SPACE: TR/TE=2500/123ms; IR SPACE: TR/TE/TI=6250/345/2100ms; T2IR SPACE: TR/TE=2500/92ms, the T2IR module: τ=100ms, TI=940ms. In patient study, only T2IR SPACE and T2 SPACE were performed due to the time limit. Data Analysis: In the volunteer study, all images were loaded to a Siemens workstation (Leonardo, Siemens Healthcare, Germany) for image analysis. Signal-to-noise ratio (SNR) and contrast ratio (CR) between vessel wall and adjacent CSF were measured using region-of-interest (ROI) analysis. SNR is defined as SNR = S/σ, where S is the mean signal intensity of a tissue (vessel wall or CSF), σ is the standard deviation of the noise region from the artifact free air region nearby. The contrast ratio between vessel wall (VW) and CSF is defined as: CR = SVW / SCSF.RESULTS
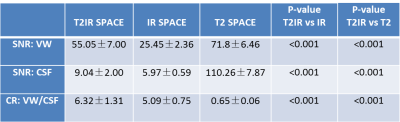
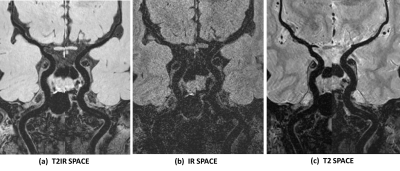
The MR scans were successfully acquired in all healthy volunteers. Figure 2 shows representative images acquired with these three seuqnce with the same resolution, coverage and scan time in one volunteer. Results of the image analysis are summarized in Table 1. As expected, the SNR of vessel wall and CSF from T2 SPACE was the highest among the three sequences (VW: 71.8±6.46; CSF: 110.26±7.87), while T2IR SPACE showed the best image contrast between vessel wall and CSF(CR: 6.32±1.31). IR SPACE led to lowest SNRCSF, but the IR pulse resulted in poor conspicuity of the vessel wall with 65% SNRVW reduction compared with T2 SPACE (IR vs T2: 25.45±2.36 vs 71.8±6.46, p<0.001). T2IR SPACE achieved similar CSF signal reduction as IR SPACE, with only 24% vessel wall signal reduction compared with T2 SPACE (T2IR vs T2: 55.05±7.00 vs 71.8±6.46, p<0.001). Figure 3 compared T2IR SPACE and T2 SPACE in a stroke patient.DISCUSSION AND CONCLUSION:
In this work, we developed a new whole-brain T2-weighted intracranial arterial wall imaging sequence with CSF suppression. The CSF signal is high in traditional T2-weighted SPACE sequence, which makes it difficult to differentiate the outer boundary of the intracranial vessel wall and this would interfere the accurate diagnosis by the radiologist. The IR pulse reduces the CSF signal but suffers from significant SNR reduction. The T2IR preparation module used in this study suppresses CSF signal remarkably without much SNR loss compared with IR pulse. Our preliminary results suggest that T2IR SPACE has the potential as a alternative T2-weighted sequence for assessment of intracranial vascular diseases.Acknowledgements
This study was funded by National Natural Science Foundation of China (Grant Number:81830056 and 81801691)References
1. Zhang L, et al. High resolution three dimensional intracranial arterial wall imaging at 3T using T1 weighted SPACE. Magnetic resonance imaging 2015;33(9):1026-1034.
2. Mossa-Basha M, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke 2015;46(6):1567-1573.
3. Ryu CW, et al. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovascular diseases 2009;27(5):433-442.
4. Dieleman N, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation 2014;130(2):192-201.
5. van der Kolk AG, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke; a journal of cerebral circulation 2011;42(9):2478-2484.
6. Wong EC, et al. T(1) and T(2) selective method for improved SNR in CSF-attenuated imaging: T(2)- FLAIR. Magnetic resonance in medicine 2001;45(3):529-532.
7. van der Kolk AG, et al. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. European radiology 2013;23(11):2996-3004.
8. Fan Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid- attenuated T1-weighted 3D turbo spin echo. Magnetic resonance in medicine 2017;77(3):1142-1150.
Figures