0954
Accelerated Spectral-Editing MRSI using Subspace Modeling, Multi-Slab Acquisition and 3D CAIPIRINHA Undersampling1Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 2Department of Nuclear Medicine, West China Hospital, Sichuan University, Chengdu, China, 3Department of Biomedical Engineering, Tsinghua University, Beijing, China, 4Department of Engineering Physics, Tsinghua University, Beijing, China
Synopsis
Spectral-editing MRSI allows reliable and selective detection of many important metabolites, e.g., GABA and 2-HG, by eliminating all uncoupled resonances in the J-difference spectrum. Despite significant advances in fast MRSI sequences and constrained image reconstruction, spectral-editing MRSI is still limited by its long acquisition time and low spatial resolution. Recently, a subspace-based approach has been proposed to accelerate spectral-editing MRSI, reporting encouraging phantom and in vivo results. In this work, we propose to further accelerate the subspace-based spectral editing MRSI using (i) multi-slab acquisition to maximize the time efficiency of long-TR spin-echo acquisition and (ii) a 3D (2D spatial + 1D spectral) CAIPIRINHA (Controlled Aliasing in Parallel Imaging Results in Higher Acceleration) scheme for sparse sampling of the (k,t)-space. We evaluate the performance of the proposed method using simulation, phantom, and in vivo studies.
Introduction
Spectral-editing MRSI allows reliable and selective detection of many important metabolites, e.g., GABA and 2-HG, by eliminating all uncoupled resonances in the J-difference spectrum $$$^{1-3}$$$. Despite significant advances in fast MRSI sequences and constrained image reconstruction, spectral-editing MRSI is still limited by its long acquisition time and low spatial resolution $$$^{4-6}$$$. Recently, a subspace-based approach has been proposed to accelerate spectral-editing MRSI, reporting encouraging phantom and in vivo results $$$^{7}$$$. In this work, we propose to further accelerate the subspace-based spectral editing MRSI using (i) multi-slab acquisition to maximize the time efficiency of long-TR spin-echo acquisition and (ii) a 3D (2D spatial + 1D spectral) CAIPIRINHA (Controlled Aliasing in Parallel Imaging Results in Higher Acceleration) scheme for sparse sampling of the (k,t)-space.Methods
We propose a multi-slab 3D, semi-LASER, spectral-editing sequence for data acquisition. As shown in Fig. 1, two 3D-slabs are acquired per TR, which allows 2x acceleration of imaging speed while keeping high-SNR efficiency of true 3D encoding. Conventional refocusing pulses are replaced by high-bandwidth adiabatic refocusing pulses $$$^{8}$$$ to improve the accuracy of spatial selectivity (as shown in Fig. 2). Compared to the conventional semi-LASER sequence that uses two pairs of adiabatic pulse for spatial selective excitation $$$^{9}$$$, the proposed sequence only applies two adiabatic pulses to the slice direction for improved spatial coverage and reduced SAR. Echo-plannar spectroscopic imaging readouts and blipped phase encodings are used to enable simultaneous spectral encoding and spatial encoding in two directions.
We propose to further accelerate the data acquisition speed of the sequence in Fig. 1 by incorporating parallel imaging to the subspace framework and by using a 3D (2D spatial + 1D spectral) CAIPIRINHA scheme for sparse sampling of the (k,t)-space. As shown in Fig. 3a, this scheme extends the 2D periodic lattice sampling of the k-space in the conventional CAIPIRINHA method $$$^{10-11}$$$ to 3D periodic lattice sampling of the (k,t)-space for fast MRSI. The proposed 3D CAIPIRINHA undersampling results in sheared aliasing patterns in the (x,f)-space, which further improves the g-factor of parallel imaging. This sampling pattern suits particularly to the subspace-based image reconstruction framework (as shown in the simulation results in Fig. 3b) because the aliasing along the frequency domain can be easily resolved with help of spectral basis functions that can be learned from the physical model of metabolite spectrum and/or "training" data $$$^{12-13}$$$.
We use a subspace-based approach for image reconstruction. We first use the union-of-subspace method $$$^{14}$$$ to remove the residual water signal and the unsuppressed subcutaneous lipid signal from the MRSI data. We write the spatial-spectral distribution of J-difference spectrum of metabolites as partially separable functions $$$^{15}$$$: $$\rho_{DIFF}(x,f)=\sum_{n=1}^{N} u_{DIFF,n}(x) v_{DIFF,n}(f), (1)$$ where $$$u_{DIFF,n}(x)$$$ and $$$v_{DIFF,n}(f)$$$ are the corresponding spatial and spectral basis functions. We estimate the spectral basis functions by leveraging the physical model of metabolite spectrum and subject-adaptive information from a low-resolution training. We then reconstruct MRSI images (or determine the spatial coefficients $$$u_{DIFF,n}(x)$$$) by fitting the model in Eq. (1) to the sparsely sampled (k,t)-space data. Sensitivity information is incorporated to the forward model and anatomical constraints are used for better image reconstruction $$$^{12-13,16-18}$$$. The spatiotemporal distribution of the imaging object when the spectral editing pulse is turned off can be reconstructed similarly.
Results and Discussions
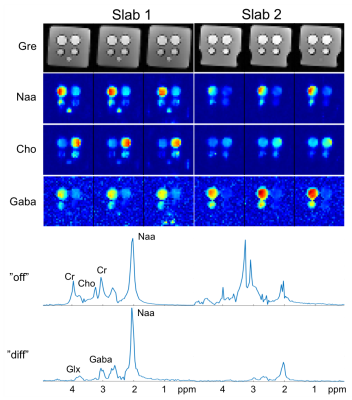
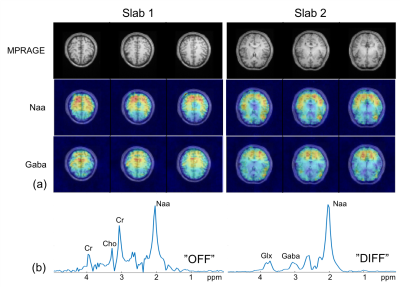
We evaluated the performance of the proposed method using simulation, phantom, and in vivo studies. Figure 2 shows the improved spatial-selectivity of the designed adiabatic refocusing pulse over the conventional pulse in a water phantom experiment. The simulation results in Fig. 3 illustrate the benefits of the proposed 3D CAIPIRINHA undersampling scheme in terms of SNR for the subspace-based reconstruction of MRSI images from sparsely sampled (k,t)-space data. Figures 4 and 5 show GABA edited MRSI results obtained from phantom and healthy human subject experiments (approved by our local IRB) on a 3T MR scanner. The two experiments were performed using the sequence in Fig.1 with the same imaging parameter: 64x64x12 spatial encodings for each slab, 4-mm isotropic resolution, 300 spectral encodings, and TR/TE=1200/68 ms. The (k,t)-space data were retrospectively undersampled by a factor of 3, resulting a total acceleration factor of 6 (2x acceleration from multi-slab acquisition). A low-resolution CSI experiment was performed in a 3-min acquisition to obtain "training" data for the subspace reconstruction. As can be seen, the proposed method produced high-resolution metabolite maps and good-quality spectra.Conclusions
We present a novel method for accelerated high-resolution spectral editing MRSI, which is enabled by subspace modeling, multi-slab 3D semi-LASER acquisition, and 3D CAIPIRINHA undersampling of the (k,t)-space. It could make high-resolution spectral-editing MRSI practical for clinical applications.Acknowledgements
This work was partially supported by the National Institutes of Health (T32EB013180, R01CA165221, R21EB021710, and P41EB022544), the Federal Share of Program Income earned by MGH on C06 CA059267, and National Natural Science Foundation of China (81471692).References
1. Puts NAJ and Edden RAE. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Progress in Nuclear Magnetic Resonance Spectroscopy, 2012;60:29-41.
2. Mullins PG, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 2014;86:43-52.
3. Andronesi OC, et al. Detection of 2-Hydroxyglutarate in IDH-Mutated Glioma Patients by In Vivo Spectral-Editing and 2D Correlation Magnetic Resonance Spectroscopy. Science Translational Medicine, 2012;4:116ra4.
4. Bogner W, et al. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. Neuroimage 2014;103:290-302.
5. Zhu H, et al. High Resolution Spectroscopic Imaging of GABA at 3 Tesla. Magnetic Resonance in Medicine 2011;65:603-609.
6. Dydak U, et al., High-Speed GABA Mapping in Human Brain with MEGA-PEPSI at 3 Tesla. In Proceedings of ISMRM 2010, p. 916.
7. Ma C, et al. MEGA-SPICE: A subspace-based approach to accelerated high-resolution spectral edited MRSI. In Proceedings of ISMRM 2018, p. 1060.
8. Garwood M, et al. The return of the frequency sweep: Designing adiabatic pulses for contemporary NMR. J Magnetic Resonance, 2001;153:155–177.
9. Scheenen TWJ, et al. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magnetic Resonance in Medicine 2008;59:1-6.
10. Breuer FA, et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magnetic Resonance in Medicine 2005;53:684–691.
11. Breuer FA, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magnetic Resonance in Medicine 2006;55:549–556.
12. Lam F and Liang ZP. A subspace approach to high-resolution spectroscopic imaging. Magnetic Resonance in Medicine 2014;71:1349-1357.
13. Lam F, et al. High-resolution 1H-MRSI of the brain using SPICE: Data acquisition and image reconstruction. Magnetic Resonance in Medicine 2016;76:1059-1070.
14. Ma C, et al., Removal of Nuisance Signals from Limited and Sparse 1H MRSI Data Using a Union-of-Subspaces Model. Magnetic Resonance in Medicine 2016;75:488-497.
15. Liang ZP. Spatiotemporal imaging with partially separable functions. In Proc. IEEE ISBI, USA, 2007;988-991.
16. Ma C, et al. High-resolution 1H-MRSI of the brain using short-TE SPICE. Magnetic Resonance in Medicine 2017;77:467-479.
17. Ma C, et al. High-Resolution Dynamic 31P-MRSI Using a Low-Rank Tensor Model. Magnetic Resonance in Medicine 2017;78:419-428.
18. Peng X, et al. Simultaneous QSM and metabolic imaging of the brain using SPICE. Magnetic Resonance in Medicine 2017. DOI: 10.1002/mrm.26972.
Figures