0944
3D-EPTI for Ultra-fast Multi-contrast and Quantitative Imaging1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 3Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States
Synopsis
A new technique, 3D-EPTI with inversion-recovery-prepared gradient- and spin-echo readouts, was developed to rapidly acquire a time-series of distortion- and blurring-free multi-contrast images. The technique relies on a new ‘time-resolved’ multi-shot 3D-EPI readout with a hybrid spatio-temporal CAIPI and golden-angle radial-blade sampling scheme. With just about 2 minutes of 3D-EPTI acquisition, thousands of brain volume data at 1.1 mm isotropic resolution can be generated at different TIs and TEs, capturing signal evolution of T1 inversion recovery interspersed with T2/T2* decay, enabling rapid simultaneous quantitative parameters estimation.
Introduction
Echo Planar Time-resolved Imaging (EPTI)[1] is an efficient distortion/blurring-free multi-shot EPI technique for multi-contrast/quantitative imaging. It has been demonstrated to provide hundreds of images at different TEs with sub-millisecond temporal increments using only a small number of EPTI shots, together with high-quality T2, T2*, tissue phase, and susceptibility weighted imaging.
In this study, we extend the EPTI concept to 3D k-space encoding and volumetric acquisition (3D-EPTI), with the aim of achieving rapid high-SNR isotropic resolution quantitative imaging. The 3D-EPTI readout is inserted into an inversion-recovery sequence with a combined gradient- and VFA-GRASE (variable-flip-angle gradient and spin-echo)[2,3] acquisition, to provide imaging time-series with mixed T1, T2, and T2* weightings. To achieve high encoding acceleration, a hybrid spatio-temporal CAIPI[1] and golden-angle[4] radial-blade sampling is developed. A subspace reconstruction[5,6,7] with locally low-rank constraint is then used to recover thousands of high isotropic resolution 3D brain images at different TIs and TEs from such dataset, together with their quantitative parameters using about 2 minutes of scan data.
Methods
Theory: As illustrated in Fig. 1a, in each TR, an inversion pulse followed by a small-flip-angle GE train and a VFA-GRASE readout train is applied to obtain EPTI-encoded signals, with T1 recovery interspersed with T2* and T2 decays. Each small-flip-angle/VFA RF-pulse is followed by an EPTI readout, which covers a small ky-kz block of the 3D k-space using a zigzag trajectory[1] with an interleaved acceleration in phase/partition-encoding direction as illustrated in Fig. 1b. Such spatio-temporal CAIPI trajectory enables effective use of coil sensitivity information during the reconstruction of the highly-undersampled ky-kz-t block (e.g., Ryblock×Rzblock=12×6, for 72 phase&partition encodings per 3D-EPTI readout). For each TR, a series of such EPTI shots are acquired at different TIs (Fig. 1a). Across multiple TRs, ky-kz blocks acquired at the same TI form a diagonal radial-blade in ky-kz space, with different blade angulation used for different TIs to compose a golden-angle radial-blade Cartesian sampling pattern (Fig. 1c). This creates favorable spatio-temporal incoherent aliasing for constrained reconstruction and permits further acceleration through acquiring only 1-2 blade per TI.
A subspace reconstruction is used to recover the images at different TIs and TEs. In this reconstruction, possible signal evolution curves are first generated using tissue and acquisition parameters to extract subspace bases, which can then be used to represent the actual signal evolution[5]. To further improve the conditioning and SNR of the reconstruction, locally low-rank constraint is employed. A pixel-wise matching is used to obtain the quantitative maps.
Simulation validation: To validate the ability of 3D-EPTI in obtaining multi-contrast images and quantitative maps, a simulation was conducted. An EPTI-encoded data experiencing an inversion recovery, a small-flip-angle GE train and a VFA-GRASE train was simulated using Bloch simulation and extended phase graph with Gaussian noise added. 1.1mm isotropic 3D brain data was simulated with 32-channel coil, where 3D-EPTI readouts across 19×2 TRs were performed to provide 2-radial-blade encoding per TI, resulting in a ~2-minute total scan time. The reconstructed images/maps were compared with reference maps used for the simulation.
In-vivo validation: Data were acquired on a healthy volunteer at 3T with a 32-channel receiver-array using 1.1 mm 3D-brain EPTI acquisition with an inversion recovery and a small-flip-angle train (with VFA-GRASE still to be incorporated). EPTI data across all ky-kz blocks were acquired (20.5 minutes) and retrospectively undersampled to the proposed golden angle sampling pattern with a single blade to achieve an effective ~1minute scan time. (FOVx,y,z=240×240×132 mm3, Ryblock×Rzblock=12×6; full block-sampling=19×19=361 blocks, single-blade sampling=19 blocks; Total acceleration=12×6×19=1368×).
Results
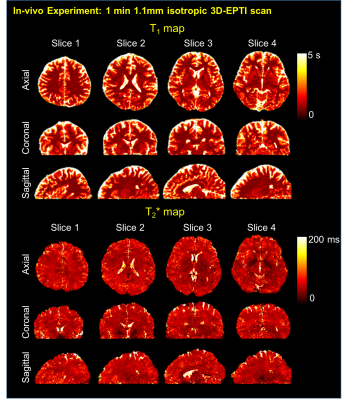
In simulation, 3D-EPTI achieves small image reconstruction errors at all TIs and TEs, and the reconstructed signal evolution curve agrees well with the reference, which then provides high-quality quantitative T1, T2, T2* maps (Fig. 2). In in-vivo experiment, the 1-minute 3D-EPTI acquisition not only provided high-quality images at different TIs and TEs (Fig. 3), but also produced comparable quantitative T1 and T2* maps to block-fully-sampled EPTI data (Fig. 4) that requires 19× longer scan time. The 3D-brain quantitative maps in three orthogonal views are shown in Fig. 5.Discussion and Conclusion
3D-EPTI is an efficient acquisition/reconstruction technique for multi-contrast and quantitative imaging. We demonstrated its ability to obtain a time series of distortion- and blurring-free images with different contrasts, together with their corresponding quantitative maps, at a high isotropic resolution across the brain in about 2-minute. Work is underway to implement the VFA-GRASE component of the proposed sequence and optimize the acquisition’s flip-angles and readout lengths using systematic CRB approach as in [8]. The ultra-fast high-resolution acquisition enabled by 3D-EPTI should pave the way for future clinical usage of quantitative mapping.Acknowledgements
This work was supported in part by NIH research grants: R01MH116173, R01EB020613, R01EB019437, U01EB025162, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043.References
1. Wang F, Dong Z, Reese T, Wald L, Setsompop K. Echo Planar Time-resolved Imaging. In Proceedings of the 26th Annual Meeting of ISMRM, Paris, France, 2018. p 0217.
2. Koichi O, and Feinberg DA. GRASE (gradient‐and spin‐echo) imaging: a novel fast MRI technique. Magn Reson Med. 1991;20(2): 344-349.
3. Kemper VG, De Martino F, Yacoub E, Goebel R. Variable flip angle 3D‐GRASE for high resolution fMRI at 7 tesla. Magn Reson Med. 2016;76(3):897-904.
4. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE transactions on medical imaging. 2007 Jan;26(1):68-76.
5. Liang, Zhi-Pei. "Spatiotemporal imagingwith partially separable functions." Biomedical Imaging: From Nano to Macro, 2007. ISBI 2007. 4th IEEE International Symposium on. IEEE, 2007.
6. Tamir JI, Uecker M, Chen W, Lai P, Alley MT, Vasanawala SS, Lustig M. T2 shuffling: Sharp, multicontrast, volumetric fast spin‐echo imaging. Magn Reson Med. 2017;77:180-195.
7. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo Planar Time-resolved Imaging (EPTI) with Subspace Constraint and optimized k-t trajectory. Submitted to: In Proceedings of the 27th Annual Meeting of ISMRM 2019.
8. Zhao, Bo, et al. "Optimal experiment design for magnetic resonance fingerprinting: Cramer-Rao bound meets spin dynamics." IEEE transactions on medical imaging (2018).
Figures