0928
Inter-frame phase alignment for Echo Planar Imaging calibration data acquired with opposite read-out polarities1Siemens Medical Solutions USA, Boston, MA, United States, 2Siemens Shenzhen Magnetic Resonance, Shenzhen, China, 3Brigham and Women's Hospital, Boston, MA, United States, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Echo-planar imaging is subject to Nyquist ghosting due to its use of fast gradient switching. To correct for this artifact, several methods have been proposed that involve a pair of calibration imaging frames acquired with opposite readout polarities. We show that a small, relative shift in the phase-encoding direction often exists between these calibration frames that may lead to compromised image quality. A simple method is proposed to account for the shift and it is shown to be effective at correcting residual Nyquist ghost in the dual-polarity GRAPPA approach. This is demonstrated in in vivo and phantom data from 3T and 7T.
Introduction
Echo-planar imaging1 (EPI) plays an important role in MRI studies in both research and clinical settings due to its high data acquisition speed. In general, EPI is prone to Nyquist ghosting due to its use of rapidly alternating readout directions, and this artifact can be exacerbated in acquisitions using accelerated parallel imaging. Correcting this imaging artifact often requires additional scans to calibrate the phase and frequency changes related to this gradient switching2. Recently, new techniques have been introduced that utilize a pair of calibration frames consisting of opposite readout polarities3-6. In this work we demonstrate that relative phase errors may exist between the pair of calibration scans that could lead to degraded ghost correction. A simple phase correction method is proposed and is shown to help improve image quality when used with the dual-polarity GRAPPA (DPG) ghost correction method5.Methods
In vivo human data were acquired at both 3T and 7T (MAGNETOM Skyra and MAGNETOM Terra, both from Siemens Healthcare, Erlangen, Germany). The 3T scans included a 32-channel receive coil and an anthropomorphic head phantom; 7T scans included a one-channel transmit, 32-channel receive coil (Nova Medical LLC, United States) and a spectroscopy phantom provided by the scanner manufacturer. Written informed consent was obtained from the human volunteers prior to scanning. All data were acquired using a prototype spin-echo-based EPI diffusion sequence with GESTE4 calibration of coil sensitivity. Imaging details: 2 mm isotropic resolution, imaging matrix = 96×96, bandwidth = 1302 Hz/pixel, 12 slices, 2.7 s TR, 63 ms TE, in-plane acceleration factors between 2 and 4.
The calibration data acquisition scheme is illustrated in Fig. 1. Each complete fully-sampled k-space data set is referred to as a calibration “frame”, characterized by one of the two readout polarity “orderings” (+-+… or -+-…). The calibration data for each slice consists of two frames with both readout orderings.
For image reconstruction, standard local-phase-corrections7 (LPC) Nyquist ghost correction was first applied in the hybrid x-ky domain, as is conventional, to the calibration data frames. Then, the proposed phase alignment is applied along the kx-y hybrid domain for each pair of calibration frames. Briefly, the central kx=0 “vertical” lines were first used as the “navigators” to extract phase differences between the pair of calibration frames as a linear function8 of the position along y. Then, the phase difference is applied to all the vertical lines in the kx-y domain. The phase-aligned frames were then used to extract the RO+ and RO‒ data as described in Fig. 1, which were further used to generate the GESTE4 calibration data. The rest of the steps are identical to the DPG training and reconstruction5. Conventional EPI with LPC based on a single calibration frame was also acquired for comparison. For simplicity only the results with b=0 and acceleration factor of 3 are shown in Results.
Results
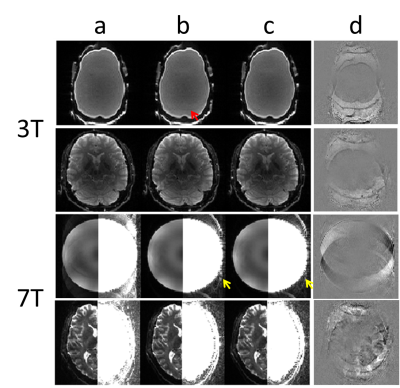
Figure 2 shows the strong artifact in the RO+ image of the calibration scans without the inter-frame phase alignment (a), the phase difference along y between the two calibration frames (b,c), and the substantially improved RO+ image with inter-frame phase alignment (d). Figure 3 shows representative reconstructed phantom and human brain images at 3T and 7T with acceleration factor of 3. Artifacts are visible for the phantom at 3T, as expected from the artefactual calibration image shown in Fig. 2(a). For the 7T results, the image quality improvement using inter-frame phase alignment is demonstrated by the subtle but reduced artifact outside the object (yellow arrows) as well as in the difference images (d).Discussion
The demonstrated linear phase ramp (Fig. 2(b)) in the image domain corresponds to a shift in the k-space along the PE direction. Correcting this linear phase ramp along y using a novel application of standard linear phase correction—normally performed in x-ky hybrid space and here applied in kx-y hybrid space—removed the ghost artifact in the calibration data and thereby improved the reconstruction of the accelerated data. Since the phase difference is prominent along y in our spin-echo-based EPI data and it is minimal in our gradient-echo-based EPI data (not shown), we suspect it is related to the eddy-current from the initial read-out gradient9. In addition to correcting for Nyquist ghosting in the two-frame calibration data, the method could also determine the amount of ky shift in k-space, whereby reducing the corresponding phase errors in the image domain and improving e.g. EPI-based phase mapping.Acknowledgements
This work was supported in part by the NIH NIBIB (grants P41-EB015896, R01-EB019437, and R03-EB023489), by the BRAIN Initiative (NIH NIMH grant R01-MH111419), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-OD010364 and S10-RR019371.References
1. Stehling MK, Turner R, Mansfield P, Echo-planar imaging: magnetic resonance imaging in a fraction of a second. Science 254(1991):43-50
2. Bruder H, Fischer H, Reinfelder HE, Schmitt F, 1992. Image reconstruction for echo planar imaging with non-equidistant k-space sampling. Magn Recon Med, 39:606-614
3. Chen NK, Wyrwicz AM. 2004. Removal of EPI Nyquist ghost artifacts with two-dimensional phase correction. Magn Reson Med 51:1247-1253
4. Hoge WS, Tan H, Kraft RA. 2010. Robust EPI Nyquist Ghost Elimination via Spatial and Temporal Encoding. Magn Reson Med 64:1781-1791
5. Hoge WS, Polimeni JR. 2016b. Dual-Polarity GRAPPA for Simultaneous Reconstruction and Ghost Correction of Echo Planar Imaging data, Magn Reson Med 76:32-44
6. Hoge WS, Setsompop K, Polimeni JR. 2018. Dual-polarity slice-GRAPPA for concurrent ghost correction and slice separation in simultaneous multi-slice EPI, Magn Reson Med, DOI: 10.1002/mrm.27113
7. Feiweier T, 2013. Magnetic resonance method and apparatus to determine phase correction parameters. U.S. patent 8,497,681.
8. Ahn CB, Cho ZH. 1987. A new phase correction method in NMR imaging based on autocorrelation and histogram analysis. IEEE Trans Med Imaging, 6: 32-36.
9. Grieve SM, Blamire AM, Styles P. 2002. Elimination of Nyquist Ghosting Caused by Read-out to Phase-encode gradient cross-terms in EPI. Magn Reson Med 47:337-343
Figures