0801
Simultaneous Multislice MRI Temperature Imaging with a Single Receiver Coil1Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
The incoherent controlled aliasing SMS method is introduced which increases volume coverage in real-time MR thermometry by acquiring multiple slices simultaneously, and requires only one receiver coil. RF pulses flip the slice phases between TRs, creating incoherent hotspot aliasing. Unaliased heating maps are then recovered from each slice using a sparsity-promoting temperature reconstruction.
Introduction
Magnetic resonance imaging-guided focused ultrasound (MRgFUS) is a non-invasive surgical technique with many applications including the treatment of neurological conditions. During MRgFUS ablative treatments, MR thermometry is used to monitor heating over a volume of tissue for dosimetry and safety. However, limited frame rates and volume coverage of current MR thermometry methods are roadblocks to many emerging applications, especially in the brain. Parallel imaging is a common way to overcome these issues, but is not feasible for MRgFUS since the FUS transducer obstructs coil placement. Here, we introduce the incoherent controlled aliasing simultaneous multislice (SMS) method, an adaptation of the SMS-controlled aliasing in parallel imaging (CAIPI) method for thermometry1 that increases volume coverage by acquiring multiple slices simultaneously, but requires only one receiver coil.Materials and Methods
Figure 1a illustrates conventional SMS-CAIPI and the incoherent controlled aliasing SMS method for a two-slice acquisition. In both acquisitions, two slices are excited simultaneously, and RF pulses are used to flip the second slice’s phase relative to the first (Figure 1b). In incoherent controlled aliasing SMS (Figure 1a, bottom), the flips are random, so the second slice is incoherently aliased in the superimposed image. Since its hotspot phase shift is smeared, a sparsity-promoting reconstruction is able to suppress the aliasing and reconstruct sparse hotspots in both slices by fitting slice-resolved baseline/pre-treatment images to a single coil’s aliased heating image signal. A SMS extension of the k-space hybrid multibaseline and referenceless algorithm2 is used for this. It uses l1 regularization to promote temperature sparsity, reflecting prior knowledge that FUS heating is focal.
Two- and three-slice simulations were run in MATLAB R2015a (Mathworks, Naticks, MA), with 128 x 128 matrix size, Gaussian-shaped hotspots (8 voxel FWHM) with peak magnitudes of 18 °C heat rises at 3T, a single receive coil with uniform sensitivity, and SNR = 50. Simulations evaluated the maximum number of simultaneous slices that could be achieved along with sensitivity to hotspot position, hotspot size and the sparsity regularization parameter.
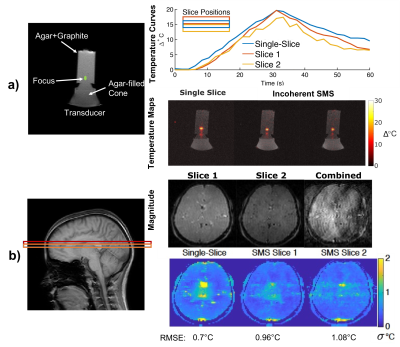
A two-slice incoherent controlled aliasing SMS pulse sequence was implemented on a 3T Philips Ingenia scanner (Philips Healthcare; Best, Netherlands). To validate the scan with FUS heating, the sequence was used to image an agar and graphite phantom that was placed on a Sonic Concepts H-101MR transducer (Sonic Concepts, Bothell, Washington, USA) (Figure 4a, left) with 128 x 128 matrix size, 16.1 ms TR, 12 ms TE, 30 dynamics, 19.2 x 19.2 cm2 FOV, 1.6 mm slice thickness. After six seconds of baseline scanning, FUS heating was manually switched on for 24 seconds. To verify the precision of the method in vivo, brain temperature maps were obtained from a healthy volunteer without heating with 224 x 224 matrix size, 16.1 ms TR, 12 ms TE, 200 x 200 cm2 FOV, and 1.6 mm slice thickness.
Results and Discussion
Figure 2 shows simulation results comparing conventional SMS-CAIPI and incoherent controlled aliasing SMS. With incoherent controlled aliasing, the true hotspot was reconstructed with low error because the sparsity-promoting reconstruction was able to suppress the non-sparse incoherent aliasing.
Figure 3 shows the sensitivity of the incoherent controlled aliasing reconstructions to different parameters. Incoherent controlled aliasing enables up to 3 slices’ temperature maps to be accurately reconstructed[WG1] , which cannot be done with conventional CAIPI. Furthermore, for both the 2 and 3 slice cases, the incoherent method is not sensitive to hotspot shifts in the y-direction (Figure 3b). For 2 slices, the method is not sensitive to large hotspot sizes, but for 3 slices, the reduced sparsity with large hotspot sizes (> 1/8 y-FOV) can lead to unacceptable error (Figure 3c). For 1, 2 and 3 slices, the reconstructions are insensitive to sparsity regularization parameter selection over at least 2 orders of magnitude (Figure 3d).
Figure 4a compares FUS temperature maps between single-slice and incoherent controlled aliasing SMS temperature mapping sequences. The shape and position and temporal evolution of heating is the same and there is no visible temperature aliasing. Figure 4b compares temperature precision maps between single-slice and incoherent controlled aliasing SMS temperature mapping sequences. The background precisions are comparable; the center of the single-slice map contains high errors due to PSF & blood pulsation which are somewhat blurred out in the incoherent controlled aliasing SMS maps.
Conclusion
We introduced and validated a new SMS MR thermometry method that relies on signal redundancy between baseline and heating images rather multiple receive coils to reconstruct slice-resolved temperature maps. The method shortens volume scan times for MR thermometry, which enables greater volume coverage in real-time MR temperature imaging to improve dosimetry and safety by monitoring off-target heating.Acknowledgements
This work was supported by the Vanderbilt Undergraduate Summer Research Program and the FUS FoundationReferences
1. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-Controlled Aliasing in Parallel Imaging (blipped-CAIPI) for simultaneous multi-slice EPI with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224.2.
2. Gaur P, Grissom WA. Accelerated MRI Thermometry by Direct Estimation of Temperature from Undersampled k-Space Data. Magn Reson Med. 2015;73(5):1914-1925.
Figures