0608
Measuring layer-dependent fMRI activity across layers in cognitive brain areas: challenges and capabilities1NIH, Bethesda, MD, United States
Synopsis
While recent advances in high-resolution fMRI allow for investigating activity across cortical layers, applications beyond the well-studied primary sensory and primary motor areas have been complicated by multiple technical challenges: lack of anatomical landmarks, complicated folding structure, weak signal, restricted task design, etc. The purpose of this study is to develop a scanning/stimulation/analysis setup that allows us to overcome these challenges. Our results suggest that with advanced imaging methodology, corresponding task design, and appropriate analysis strategies it is possible to reliably measure layer-dependent activity differences in cognitive brain areas, such as the dorsolateral prefrontal cortex.
Purpose
Layer-specific fMRI can address questions on directional feedforward and feedback information flow between brain areas1. Due to recent advancements in high-field MRI hardware, sequence design, and contrast mechanisms, layer-fMRI has been successfully applied in primary cortical areas (visual/auditory/motor)2-7.
Application beyond primary cortical areas, however, has been complicated by multiple technical challenges:
- The lack of anatomical landmarks for small FOV imaging.
- Unpredictable cortical folding across participants.
- Signal leakage across time and space from vessel biases.
- Demand for both: a) multiple task conditions and b) many trial averages.
- Weak signal and high noise level.
- Unclear correspondence between cortical depth and cytoarchitectonical cortical layers, in contrast to well studied primary areas.
The purpose of this study is to develop a robust data acquisition setup that can account for the above mentioned challenges. Here, the cognitive area dorsolateral prefrontal cortex (DLPFC) is used as a test bed to investigate the applicability and robustness of the proposed experimental setup. Subsequently, layer-dependent fMRI results in DLPFC can help to better understand the mechanisms of working memory processes8.
Methods
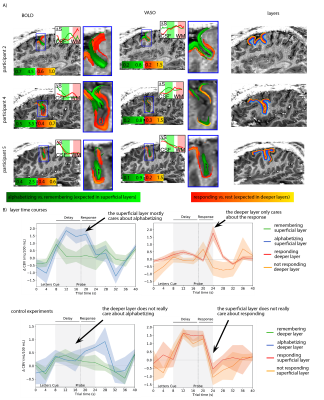
17 two-hour fMRI sessions were conducted to investigate the optimal parameter space. The functional task used to engage the superficial and deeper layers in DLPFC was a mental letter-sorting task9. Participants are briefly presented with a string of letters, then asked to either alphabetize them (manipulation condition) or remember them in the given order (maintenance condition) over a delay period. In some trials, following the delay period, participants are further asked to press a button regarding the position of a given probe letter (response condition). Different conditions are expected to differently engage the superficial or the deeper layers in DLPFC10.
We addressed the above-mentioned challenges with the following sequence and analysis choices.
- Due to the lack of clear anatomical landmarks for DLPFC, we proposed the combination of a low-resolution 6-min functional localizer with real-time analysis and a subsequent small FOV sub-millimeter layer-fMRI protocol (Fig. 1A).
- Due to the unpredictable folding pattern in cognitive brain areas, we investigated two scan orientation with different voxel anisotropies (Fig. 1BC): 0.9×0.9×1.1mm3 and 0.79×0.79×0.99mm3.
- To account for spatial leakage artifacts across layers, we used a CBV-sensitive SS-SI-VASO-3D-EPI sequence with high spatial specificity7,11-12.
- Contrasts between tasks conditions across time (which require higher temporal resolution) and layer analyses (which require high spatial resolution) were conducted in separate respectively-optimized scan protocols.
- We investigated the noise amplification through the analysis pipeline by applying signal averaging before and after the application of non-linear models (Fig. 2).
Data were acquired on a SIEMENS 7T: further scan parameters are available on GitHub: https://github.com/layerfMRI/Sequence_Github/tree/master/DLPFC_Emily. Laminar analyses were conducted in EPI space in the open software package LAYNII: https://github.com/layerfMRI/LAYNII.
Results and Discussion
- We find that despite the complicated and heterogeneous folding pattern in cognitive brain areas, the activation location is highly consistent across experiments. Thus, the online functional localizer allows us to effectively position the high-resolution imaging FOV for axial-like and sagittal-like slice orientations (Fig. 1).
- To minimize the impact of low-frequency noise, we find that it is beneficial for to refrain from event-related paradigms with random trial type ordering (Fig. 2A). Instead, pseudo-random event timings should be chosen that do not have long gaps between trials of any one type.
- Due to the weak responses and the high noise level, noise amplification effects can be challenging. We find that it is beneficial to apply signal averaging as early as possible in the analysis pipeline (Fig. 2B). Note that this kind of averaging comes along with a smaller flexibility of the task design. As such, it prohibits the application of event-related designs that rely on assumed HRFs and it is not applicable for resting-state analyses.
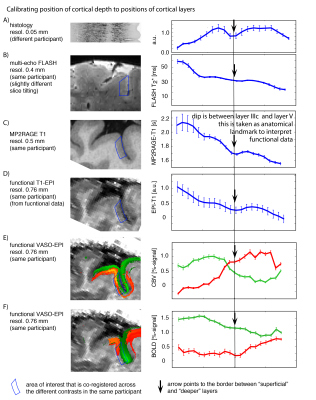
- Locations of relative cortical depth can be referred to cytoarchitectonically defined cortical layers, by means of calibrating T1-related myelin density profiles to ex-vivo data (Fig. 3).
- As previously shown in other studies14 we find that CBV results -while being noisier- can capture layer-dependent activity with higher localization-specificity (Fig. 3EF, 4A). This is due to its insensitivity to large draining veins.
Based on the results shown in Fig. 1-3, we could find a robust experimental setup to measure layer-dependent information reliably across cortical depth and across time (Fig. 4).
Conclusion
The data presented here suggest that layer-dependent fMRI in cognitive brain areas can be challenging. However, with appropriate choices in task design, sequence setup, and analysis strategies, these challenges can be addressed and reliable layer-dependent fMRI results can be obtained across space and time.Acknowledgements
This research is supported by the NIMH Intramural Research Program. We thank Dimo Ivanov and Benedikt Poser for his help with implementing the 3D-EPI ASL/VASO sequence. We thank Sriranga Kashyap for help with optimal parameters of registration in ANTS. This research is supported by the NIMH Intramural Research Program. We thank Kenny Chung and Harry Hall for radiographic assistance. The study was approved under NIH Combined Neuroscience Institutional Review Board protocol #93-M-0170 (ClinicalTrials.gov identifier: NCT00001360). Portions of this study used the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD (biowulf.nih.gov).
References
- 1 Felleman and Van Essen, Cerebral Cortex, 1: 1-113, Distributed hierarchical processing in the primate cerebral cortex.
- 2 Polimeni et al., 2010, NeuroImage, 53: 1334-1346, Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1.
- 3 Kay, K., Jamison, K., Vizioli, L., Zhang, R., Margalit, E., and Ugurbil, K. (2018). A critical assessment of data quality and venous effects in ultra-high-resolution fMRI. BioRxiv 1–45.
- 4 De Martino, F., Moerel, M., Ugurbil, K., Goebel, R., Yacoub, E., and Formisano, E. (2015). Frequency preference and attention effects across cortical depths in the human primary auditory cortex. Proc. Natl. Acad. Sci. 112, 16036–16041.
- 5 Kok, P., Bains, L.J., van Mourik, T., Norris, D.G., and de Lange, F.P. (2016). Selective Activation of the Deep Layers of the Human Primary Visual Cortex by Top-Down Feedback. Curr. Biol. 26, 371–376.
- 6 Muckli, L., De Martino, F., Vizioli, L., Petro, L.S., Smith, F.W., Ugurbil, K., Goebel, R., and Yacoub, E. (2015). Contextual Feedback to Superficial Layers of V1. Curr. Biol. 25, 2690–2695.
- 7 Huber et al., 2017, Neuron, 96: 1253-1263, High-resolution CBV-fMRI allows mapping of laminar activity and connectivity of cortical input and output in human M1.
- 8 Finn, E.S., Huber, L., Jangraw, D.C., and Bandettini, P.A. (2018). Layer-dependent activity in human prefrontal cortex during working memory. BioRxiv 425249.
- 9 D’Esposito, M., Postle, B.R., Ballard, D., and Lease, J. (1999). Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain Cogn. 41, 66–86.
- 10 Goldman-Rakic, P.S. (1996). Regional and cellular fractionation of working memory. Proc. Natl. Acad. Sci. 93, 13473–13480.
- 11 Poser, B.A., Koopmans, P.J., Witzel, T., Wald, L.L., and Barth, M. (2010). Three dimensional echo-planar imaging at 7 tesla. Neuroimage 51, 261–266.
- 12 Lu et al., 2003, MRM, 50:63-74, Functional magnetic resonance imaging based on changes in vascular space occupancy.
- 13 Wagstyl, K., Lepage, C., Bludau, S., Zilles, K., Fletcher, P.C., Amunts, K., and Evans, A.C. (2018). Mapping Cortical Laminar Structure in the 3D BigBrain. Cereb. Cortex 1–12.
- 14 Kim et al., 2013, NMR Biomed, 26: 949-962. Cerebral blood volume MRI with intravascular superparamagnetic iron oxide nanoparticles.
Figures