0581
Glutamine Metabolism Dysregulated in PDAC induced CachexiaSantosh Kumar Bharti1, Paul T Winnard1, Raj Kumar Sharma1, Yelena Mironchik1, Michael Gilbert Goggins2,3,4, and Zaver M. Bhujwalla1,5,6
1Division of Cancer Imaging Research, The Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Medicine - Gastroenterology and Hepatology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Oncology-Gastrointestinal Cancer, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Department of Pathology-Gastroenterology and Liver Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 63Department of Radiation Oncology and Molecular Radiation Sciences, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Glutamine is one of the most abundant circulating amino acids that is critical for many fundamental functions in cancer cells, including synthesis of metabolites that maintain mitochondrial metabolism, protein synthesis, acting as a carbon source or as the primary nitrogen donor for multiple essential biosynthetic pathways, and in the activation of cell signaling. Here we have identified significant differences in glutamine in human plasma from pancreatic cancer patients that were also observed in tumor interstitial fluid from pancreatic cancer xenografts that induced cachexia. These results suggest that agents with glutaminolytic activity may be useful in treating cachexia.
Introduction
Cancer-induced cachexia accounts for approximately 20% of all cancer deaths 1. In pancreatic cancer, the syndrome affects nearly 80% of patients 2-3. A major characteristic of cachexia is the accelerated skeletal muscle and fat storage wasting causing nutrient mobilization both directly as lipid and amino acids, and indirectly as glucose derived from the exploitation of liver gluconeogenesis that reaches the tumor through the bloodstream 4 that causes significant metabolic dysregulation. Glutamine has long been observed to play an important role in cancer metabolism 5. ‘Glutamine addiction’ of cancer cells was observed as early as 1955 6. The role of glutamine and glutamate in the induction of PDAC cachexia is unexplored. While glutamine supplements in a mixture with arginine and β-hydroxyl β-methyl butyrate have been investigated in reversing cachexia, results from these studies are inconclusive 7. Here, we investigated glutamine/glutamate in human plasma from PDAC patients, and in subcutaneous fluids from normal mice and tumor interstitial fluid (TIF) from cachectic (Pa04C) and non-cachectic (Panc1) tumor bearing PDAC xenografts.Methods
Plasma from patients with PDAC (n=17), patients with benign pancreatic disease (n=12) and from healthy control subjects (n=14) were included in this study. Final diagnosis was established by histopathological evaluation of surgical specimens. For 1H MRS analysis, plasma samples were thawed and homogenized using a vortex mixer. Then 300 μL of D2O phosphate buffer saline (NaCl 0.9% in 90% D2O) was added to 300 μL of plasma. After centrifugation (12000 rpm, 5 min), 550 μL of each sample was transferred to 5 mm NMR tubes and High-resolution 1H MRS was acquired on an Avance III 750 MHz Bruker MR spectrometer using Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence with water suppression 8. Subcutaneous fluid from normal mice (n=5) and tumor interstitial fluid from cachectic (Pa04C, n=13) and non-cachectic (Panc1, n=8) tumor xenografts was collected using our previously reported method 9-10. TIF samples (~50uL) were diluted to 550uL with D2O PBS buffer for MRS analysis.Results and Discussion
As anticipated, mice with cachexia-inducing Pa04C tumors showed significant weight loss with time. We found that TIF from Pa04C tumor bearing mice showed significant changes in glutamine and glutamate (Figure 1). Quantitative analysis of metabolites are shown in a metabolic heat map (Figure 2). Glutamine to glutamate ratios quantified in human plasma showed a significant decrease in PDAC patients as compared to normal healthy controls. PDAC cells have been identified to be glutamine avid that was attributed to an increase of the ASCT2 glutamine transporter 11. Cancer cells rapidly convert glutamine to glutamate due to the high expression of mitochondrial GLS. Glutamate is metabolized to a-ketoglutarate through glutamate dehydrogenase and enters the TCA cycle for the production of pyruvate and ATP. Pharmacological inhibition of GLS was found to be effective in a subset of PDAC 12. Targeting GLS or the glutamine transporter ASCT2 may be useful in treating cachexia. A small molecule agonist of ASCT2 was recently reported that may create opportunities for therapeutic interventions 13.Acknowledgements
Supported by NIH R01CA193365 and R35CA209960.References
1. Argiles, J. M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F. J., Cancer cachexia: understanding the molecular basis. Nature reviews. Cancer 2014, 14 (11), 754-62. 2. Fearon, K. C.; Baracos, V. E., Cachexia in pancreatic cancer: new treatment options and measures of success. HPB (Oxford) 2010, 12 (5), 323-4. 3. Ozola Zalite, I.; Zykus, R.; Francisco Gonzalez, M.; Saygili, F.; Pukitis, A.; Gaujoux, S.; Charnley, R. M.; Lyadov, V., Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: A systematic review. Pancreatology : official journal of the International Association of Pancreatology 2015, 15 (1), 19-24. 4. Porporato, P. E., Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. 5. Choi, Y. K.; Park, K. G., Targeting Glutamine Metabolism for Cancer Treatment. Biomolecules & therapeutics 2018, 26 (1), 19-28. 6. Eagle, H., Nutrition needs of mammalian cells in tissue culture. Science 1955, 122 (3168), 501-14. 7. Berk, L.; James, J.; Schwartz, A.; Hug, E.; Mahadevan, A.; Samuels, M.; Kachnic, L.; Rtog, A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2008, 16 (10), 1179-88. 8. Van, Q. N.; Chmurny, G. N.; Veenstra, T. D., The depletion of protein signals in metabonomics analysis with the WET-CPMG pulse sequence. Biochemical and biophysical research communications 2003, 301 (4), 952-9. 9. Dore-Savard, L.; Lee, E.; Kakkad, S.; Popel, A. S.; Bhujwalla, Z. M., The Angiogenic Secretome in VEGF overexpressing Breast Cancer Xenografts. Scientific Reports 2016, 6, 39460. 10. Winnard, P. T.; Bharti, S. K.; Penet, M.-F.; Marik, R.; Mironchik, Y.; Wildes, F.; Maitra, A.; Bhujwalla, Z. M., Detection of Pancreatic Cancer–Induced Cachexia Using a Fluorescent Myoblast Reporter System and Analysis of Metabolite Abundance. Cancer Research 2016, 76 (6), 1441-1450. 11. Roux, C.; Riganti, C.; Borgogno, S. F.; Curto, R.; Curcio, C.; Catanzaro, V.; Digilio, G.; Padovan, S.; Puccinelli, M. P.; Isabello, M.; Aime, S.; Cappello, P.; Novelli, F., Endogenous glutamine decrease is associated with pancreatic cancer progression. Oncotarget 2017, 8 (56), 95361-95376. 12. Rajeshkumar, N. V.; Yabuuchi, S.; Pai, S. G.; De Oliveira, E.; Kamphorst, J. J.; Rabinowitz, J. D.; Tejero, H.; Al-Shahrour, F.; Hidalgo, M.; Maitra, A.; Dang, C. V., Treatment of Pancreatic Cancer Patient-Derived Xenograft Panel with Metabolic Inhibitors Reveals Efficacy of Phenformin. Clinical cancer research : an official journal of the American Association for Cancer Research 2017, 23 (18), 5639-5647. 13. Schulte, M. L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S. T.; Kondo, J.; Coffey, R. J.; Johnson, M. O.; Rathmell, J. C.; Sharick, J. T.; Skala, M. C.; Smith, J. A.; Berlin, J.; Washington, M. K.; Nickels, M. L.; Manning, H. C., Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nature medicine 2018, 24 (2), 194-202.Figures
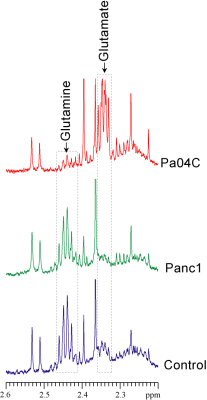
Figure 1:
Representative 1H MR spectra obtained from TIF of Panc1 (green) and Pa04C (red)
tumor bearing mice, and subcutaneous fluid (SF) (blue)
obtained by grafting a collection chamber in the subcutaneous flank space of
healthy mice. Significant metabolic differences were identified in glutamine
and glutamate between SF and TIF obtained from Panc1 and Pa04C tumors.
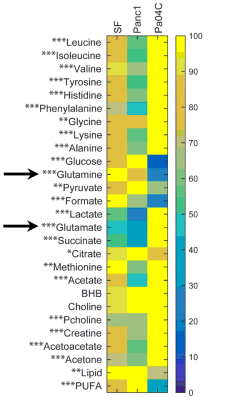
Figure
2: Metabolic heat maps showing differences in glutamine and glutamate.
Metabolites in interstitial fluid obtained from healthy controls and from Panc1
and Pa04C mice tumors. The maximum level of a metabolite present in a group was
set to 100% to which corresponding levels in the other groups were normalized.
Percentage change of metabolites in Pa04C TIF were categorized with respect to
that of Panc1 (*change >10%, **change >25%, ***change >50%).
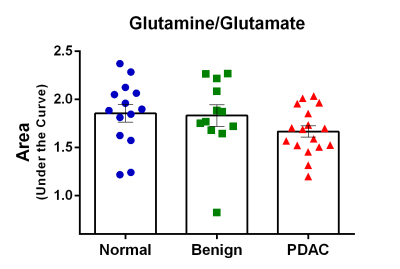
Figure 3:
Glutamine/glutamate ratio in human plasma samples. The significant decrease
(p<0.05) of the glutamine/glutamate ratio in the plasma of PDAC patients was
primarily due to an increase of glutamate, and a slight decrease of glutamine
in the plasma of these patients.