0548
Saturation transfer of relayed nuclear Overhauser enhancement: its relationship with the chemical exchange1University of Pittsburgh, Pittsburgh, PA, United States, 2Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Korea, Republic of, 3Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
In saturation transfer experiments, the Nuclear Overhauser enhancement (NOE) is often detected from mobile macromolecules and is usually assumed through an exchange-relayed pathway. However, the relationship of such relayed-NOE signals with the chemical exchange is still unclear. We reported simulation, phantom and in vivo studies to examine the dependence of
Introduction
Recent studies have shown that significant NOE signals can be detected in saturation transfer experiments, similar to the CEST effect 1-3. It has been suggested that these NOE signals are detected through an exchange-relayed pathway, i.e., saturation transfer of magnetization from the NOE pool to the bulk water must be relayed by a chemical exchange pool. However, the relationship of the relayed NOE signal with the chemical exchange, including the dependence on pH, is still controversial and not fully understood. In this work, we performed simulation, phantom and in vivo studies to examine the dependence of relayed NOE signal on chemical exchangeMaterials and Methods
Simulations:
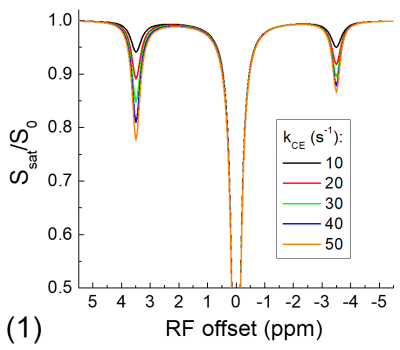
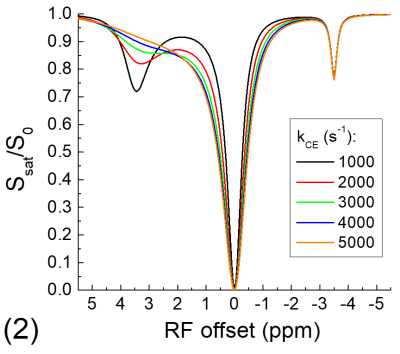
The exchange-relayed NOE signal was simulated with Block-McConnell equations for three pools: water (pool A), chemical exchange (pool B), and NOE (pool C) pools, where the exchange of magnetization only occurs between pools A and B (i.e., CE) and between pools B and C (i.e., NOE). The Larmor frequencies of pools B and C are assumed to be 3.5 and -3.5 ppm, respectively, and the rate of NOE (kNOE) was assumed to be 5 s-1. The rate of chemical exchange (kCE) is simulated for a slow chemical exchange case from 10 to 50 s-1 and a fast exchange case from 1000 to 5000 s-1. The fractions of pool B and C are assumed to be 0.003 and 0.03, and the T1 and T2 of protons for all three pools are assumed to be 2 s and 1 s, respectively.
Experiments:
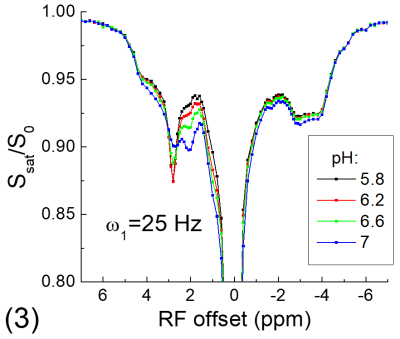
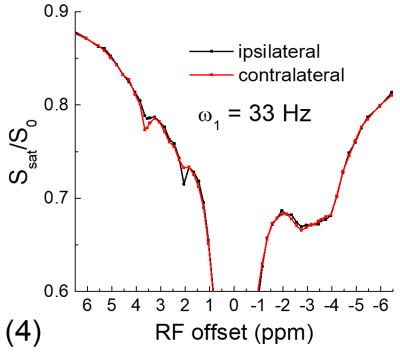
All experiments were performed on a 9.4 T MRI system. For the phantom experiment, 20% of egg white albumin was dissolved in PBS, titrated into 4 different pH values and measured at room temperature. For in vivo experiments, four Sprague-Dawley rats that underwent permanent middle cerebral artery occlusion (MCAO) were studied. CEST Z-spectra were measured at ~1 h post MCAO with spin-echo echo planar imaging, a 2.0 mm slice thickness, a field of view of 28.8 mm x 28.8 mm, and a matrix size of 64 x 64. Apparent diffusion coefficient (ADC) maps were measured to identify the ischemic core, and T1 maps were acquired with an inversion recovery sequence.
Results
Fig. 1 shows that for the slow CE case, both the NOE signals at -3.5 ppm and the CEST signals at 3.5 ppm decrease with kCE and have a similar dependence on kCE. Although the pool size of NOE is 10 times larger than that of CE, the NOE signal is always smaller than that of CEST. For the fast CE case (Fig. 2), while the CEST signals show large dependence on kCE, the NOE signals are independent of the kCE values. In egg white albumin samples (Fig. 3), there are clear pH-dependent CEST signals in the downfield frequencies, especially between ~1.5 to 3 ppm. At upfield frequencies, however, the Z-spectra show much smaller pH-dependence and almost overlaps for pH = 5.8, 6.2, and 6.6, and the NOE effect is only slightly larger for the pH =7 sample. In MCAO rats, the averaged Z-spectra (n=4, Fig. 4) obtained from ipsilateral and contralateral ROIs shows a decrease of the APT signal at 3.5 ppm and an increase of the guanidyl signal at 2 ppm, but almost overlap in the upfield frequencies. Note that at this short stroke duration, there are already significant changes in water ADC and tissue pH for the ischemic core, but only minimal variations in MT and protein concentrations 4, and the change of water T1 is only about 10%.Discussions
Our simulated data indicate that when NOE is relayed by slow chemical exchange protons (e.g., amide), it has similar pH-dependence with the CE. If NOE is relayed by fast chemical exchange protons, it is almost independent of pH. Our phantom and in vivo results confirm previous finding of our group and a few other studies and show that the aliphatic NOE signal has much smaller pH-dependence than CEST signals 3,5,6. This suggests that the NOE signals may be mostly relayed by fast exchanging protons such as amine, guanidyl, and hydroxyl protons, and the contribution from the backbone amide proton is likely very small. Our results also indicate that NOE signals can be used as a pH-insensitive index and provide novel structural information of mobile proteins.Acknowledgements
This work is supported by NIH grant NS100703.References
1. Ling W et al., Proceedings of National Academy of Sciences 105:2266 (2008).
2. Jones CK et al., NeuroImage 77:114 (2013).
3. Jin T et al., Magnetic Resonance in Medicine 69:760 (2013).
4. Zhou JY et al., Nature Medicine 9:2085 (2003).
5. Goerke S et al., NMR in Biomedicine 28:906 (2015).
6. Zhou IY et al., Magnetic Resonance in Medicine doi: 10.1002/mrm.27385, (2018).
Figures