0507
Identification of early stage murine pancreatic tumors by a combinatorial approach employing SPEN DWI, FLAIR T1 and hyperpolarized 13C MRI1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2The Moross Integrated Cancer Research Center, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Pancreatic ductal adenocarcinoma has a poor prognosis. This study explored the use of a multimodal screening approach on a preclinical mouse PDAC model that included T1 and T2 mapping, SPatiotemporal ENcoding (SPEN) and EPI-based DWI, and MT methods, and hyperpolarized 13C metabolic MRSI, to follow the progress of the disease from early on. Whereas T2 and MT were of little help, markedly decreased diffusivity, extended T1s and significantly higher metabolic activities could be detected for large and small tumors alike. These approaches could provide a translatable approach to the early noninvasive detection of pancreatic cancer, leading to timely treatment.
Introduction
Despite strides in cancer treatment, pancreatic ductal adenocarcinoma (PDAC) remains at a 5-year survival rate lower than 5% –a rate nearly unaffected for a century and which makes it the fourth major cause of cancer-related deaths.[1] This prognosis reflects PDAC’s high metastatic index, coupled to a paucity of early detection methods.[2] By contrast to what happens with other malignancies MR plays a relatively minor role in detecting PDACs, which generally lack contrast vis-à-vis surrounding tissues, and for which contrast agents do not extravasate efficiently. Evidences of differentiated pancreatic cancer metabolism have recently been demonstrated by Hyperpolarized MRSI.[3] Recent work has also shown potential correlations between diffusion MRI (DWI) and tissue fibrosis [4] –yet these measurements are uncertain when implemented using EPI in regions that, like the abdomen, are affected by motions and close to fat/air/water interfaces. The goal of this work is to explore the usefulness of emerging MRI forms, and in particular of robust DWI approaches based on SPatiotemporal ENcoding (SPEN,[5]) to develop new contrasts that can target this disease. DWI and DTI was complemented by relaxation (T1, T2) and magnetization-transfer (MT) measurements as well as with hyperpolarized 13C metabolic imaging, in the search for a combination that might serve as a toolkit for early-detection purposes. Results observed in a preclinical model are here reported.Methods
Eight male black mice bearing orthotopic PAN-2 PDAC and four naïve black control mice were scanned. 1H MRI experiments were conducted either on 7 T magnets operated by Varian consoles. 13C Hyperpolarization was conducted in an Oxford Instruments Hypersense operating at 94 GHz and 1.4K, and 13C MRSI measurements were carried out on a Bruker Biospec 4.7T scanner using a dual-tuned 1H/13C volume coil. See Captions for further details.
Results
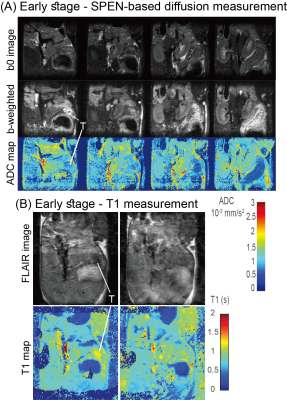
PDAC tumors could barely be identified by anatomical 1H MRI until after ca. 14 days post-implantation –a period henceforth called the early-detection stage. Different 1H MR contrasts were explored throughout this and later periods. Neither T2 nor MT weighting/mapping could identify the tumor until weeks following implantation, when it had already reached ≈1cm and was visible in the anatomic image (Figure 1). It has been reported that in humans, pancreatic tumors have lower ADCs than surrounding tissue;[6] due to the presence of motion, fat and air, however, EPI-based DWI measurements could barely observe tumors on the basis of this behavior. Robust SPEN-based DWI techniques, by contrast, could unambiguously observe in vivo PDACs in mice (Fig. 2), with isotropic diffusivities of ≈7x10-4 mm2s-1 compared to surrounding values of 1.1x10-3 mm2s-1. In addition (not shown) diffusion tensor properties with specific alignment were detected by SPEN. This agreed with more sensitive results collected in vitro on surgically extracted tumors (Fig. 3), according to which both a reduced diffusivity and kurtosis could be related to a dense, locally ordered morphology revealed for the tumorous stroma by staining and microscopy. Interestingly, the robustness of in vivo SPEN DWI experiments successfully identified the tumors with their anomalously low ADCs, when these were only ≈2 mm in diameter (Fig. 4A). The sole other 1H MRI contrast that highlighted the presence of the tumor was the T1, which when mapped lead to slightly longer values (≈1.45 s) than most of the tissues in the abdominal region (0.9 s, Figure 4B). In addition to 1H measurements, MR’s competence to detect PDAC tumors was assayed by injection of hyperpolarized 13C1-pyruvate. While 13C MRSI results were characterized by much poorer spatial resolutions than 1H counterparts, they could clearly identify the presence of even incipient tumors by a large increase in the 13C1-lactate production (Fig. 5): The most intense lactate signals tended to originate from the approximate center of the tumor, and a several-fold increase in the Lac/Pyr ratio were observed when compared against ratios arising from other abdominal regions or from controls.Discussion & Conclusion
A comprehensive multimodal exploration of contrasts for early PDAC detection was conducted in a murine model. Three potentially translatable contrast sources were identified: SPEN-based DWI/DTI, T1 mapping via Inversion Recovery, and hyperpolarized 13C MRSI. Findings from these methods could jointly reveal the presence of PDAC even at an early stage, when the tumor is small and treatable yet undetectable by anatomical 1H images. It remains to be seen whether these tools can also highlight malignancies in human cases, and whether they can distinguish malignancies from pancreatic inflammations. Research into these avenues is currently in progress.Acknowledgements
This work was supported by the Kimmel Institute for Magnetic Resonance (Weizmann Institute), the Israel Science Foundation (grants 2508/17 and 965/18), a Thompson Family Foundation grant, and the EU Horizon 2020 programme (Marie Sklodowska-Curie Grant 642773).References
[1] E.S. Lee, and J.M. Lee. World J. Gastroenterol. 20 (2014) 7864.
[2] T. Yin et al. World J. Methodol. 7 (2017) 101.
[3] E.M Serrao et. al. GUT 65 (2016) 465.
[4] N. Farr et al. Cancer Med. 6 (2017) 1082.
[5] E. Solomon et al. JMR. 236 (2013) 76.
[6] N. Nissan et al. NMR Biomed. 30 (2017) e3728.
[7] Q. Bao et. al. ISMRM (2018) 0380.
[8] Q. Bao et. al. ISMRM (2018) 1021.
[9] L. Zhao et al. J Magn Reson B. 113 (1996) 179.
Figures


Comparison of diffusion SPEN and EPI results on a fully-grown pancreatic tumor (day 11 post-implantation; tumor highlighted with T). SPEN images (4 slices) were acquired with TE=40 ms, TR =1.5-2.0 s, slice thickness=1 mm, and in-plane resolution of 200-250 µm, acquiring 5 shots and 2 averages[8]. Spin-Echo-EPI data were collected with TE=35 ms, TR=1.5-2.0 s, slice thickness=1 mm and an in-plane resolution of 250 µm, by acquiring 4 shots and 2 averages, as well as a reference scan for correcting the even/odd half-Nyquist ghost. DWI was attained by relying on a bipolar-gradient pulse module for SPEN, and with a PGSE module for EPI, b-weighting=1200 mm2s-1.

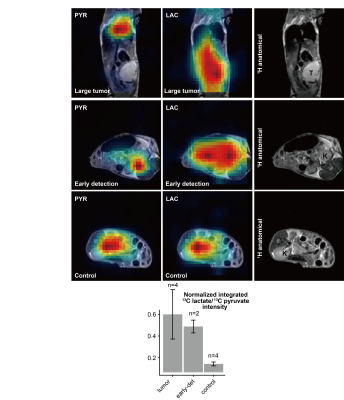
Representative 13C1-pyruvate injections following hyperpolarization with 15mM Ox63 into animals that: Had a tumor visible in anatomical images (top row). Had undergone implantation but the tumor was not identifiable in anatomical images (hence named ‘early-detection’ animals, center row). Controls (either naïve animals or animals whose tumors failed to grow, bottom row). The statistical comparison between the results in the different types of animals is demonstrated in a histogram. 13C MRSI images were recorded using a centric chemical-shift imaging sequence[9] with TR=68 ms, slice thickness=0.4-0.7 cm, in-plane matrix size=8x8, and square 3.5 cm(axial) or 4 cm(sagittal) FOVs. Measurement time=3 sec/image.These are displayed alongside anatomical FLASH images.