0375
Noninvasive Prostate Cancer Grading Using Diffusion MR Structural Fingerprints1Radiology, Washington University School of Medicine, Saint Louis, MO, United States, 2Radiology, Changhai Hospital, Shanghai, China, 3Medical Scientist Training Program, The University of Alabama at Birmingham, Birmingham, AL, United States, 4Medicine, University of Missouri – Kansas City, Kansas City, MO, United States, 5Radiology, Guangzhou First People's Hospital, Guangzhou, China, 6Radiology, The First Affiliated Hospital of Nanchang University, Nanchang, China
Synopsis
Prostate cancer (PCa) is second most common cause of cancer death among American men. Curently clinicians rely on needle biopsies for PCa grading, although biopsy Gleason scores often differ from those of pathological analyses. We demonstrated modified-DBSI captured and quantified heterogeneous diffusion fingerprints reflecting prostatic histopathology, capable of noninvasively grading PCa with high accuracy. The diagnostic power of modified-DBSI could prevent low-grade cancer patients from undergoing unnecessary and costly invasive procedures, offering patients more reliable assessments on cancer progression during active surveillance, and helping patients and clinicians to determine the most appropriate treatment options.
Introduction
Prostate cancer (PCa) is second most common cause of cancer mortality among American men. Accurate grading of PCa is imperative to properly diagnose patients, assess cancer aggressiveness, and stratify treatments. Currently, clinicians rely on needle biopsies for PCa grading, although biopsy Gleason scores often differ from those of pathological analyses. While many non-invasive grading methods have been explored, none of them can replace needle biopsies. DWI-derived ADC reported notable differences in Gleason scores between high- and low-grade PCa.1,2 However, ADC measurements alone still do not provide clinicians with sufficiently accurate information to avoid needle biopsies. Herein, we sought to utilize a novel MRI technique, i.e., refined diffusion basis spectrum imaging (DBSI) modeling and machine learning, to noninvasively grade PCa. We demonstrate modified DBSI can accurately grade PCa for stratifying treatment plan, and maybe an ideal tool for active surveillance.
Methods and Materials
Modified-DBSI models prostate diffusion-weighted MRI signals as linear combination of an anisotropic diffusion tensor and a spectrum of isotropic diffusion (Fig.1). Two-hundred sixty-four patients with clinical suspicion of PCa were recruited. Biopsy confirmed 101 PCa-positive patients. DBSI was performed on a 3T Siemens Skyra scanner, and Gleason scoring were performed on these biopsy sites. Total 101 prostatectomy specimens from additional 26 PCa patients were procured for ex vivo imaging and post-MRI histology validation (Fig.2A/B). In vivo DWIs were acquired at 2x2x4 mm3 resolution with a 25-dir icosahedral-diffusion-encoding scheme with maximum b-value=1500 s/mm2. Prostatectomy specimens were examined on an Agilent 4.7T scanner using the same diffusion-weighting scheme with maximum b-value=3000 s/mm2 to acquire DWIs at 0.5x0.5x0.5 mm3 resolution. Temperature differences between ex vivo and in vivo scans were remedied using a 1.5× scale. An experienced pathologist determined the Gleason scores of biopsy and/or prostatectomy specimens. H&E images were co-registered with ex vivo DBSI-derived structural-metrics maps from prostatectomy. Co-registered image-pairs were fed into voxel-based support vector machine (SVM) algorithm to construct predictive models to assess Gleason scores and define National Comprehensive Cancer Network (NCCN) risk groups.Results
We demonstrate the association between modified DBSI structural metrics and various pathologies in a prostatectomy specimen. Results showed lymphocytes exhibited a predominant signal fraction from highly-restricted-diffusion-tensor; PCa exhibited primary signal fraction from restricted-diffusion-tensor; stromal tissues exhibited hindered-diffusion-tensor and lumen space exhibited predominant free-diffusion-tensor (Fig.2B). Modified-DBSI diffusion-fraction-maps approximately delineated distributions and fractions of various prostatic-histopathological structures (Fig.2C).
Multiparametric MRI from a 63-year-old PCa patient with a transition-zone malignant lesion was examined. ADC distribution from the outlined tumor ranged between 0.3–0.8 µm2/ms (Fig. 3A). Modified-DBSI isotropic-diffusion-tensor ADC from the same region revealed distributions representing lymphocytes, tumor cells, stromal cells, and lumen (Fig.3B). The whole-mount H&E confirmed modified-DBSI-derived distributions of these structures (Fig.3C). ADC histogram distinguished higher-grade PCa (Grade 2-5) from benign prostate and lower-grade PCa (Grade 1) but it failed to further distinguish higher-grade tumors. Modified-DBSI-derived diffusion-metric-profiles distinguished benign tissues from malignant tissues of different tumor grades (Fig.4). Benign-peripheral-zone tissue exhibited a high free-diffusion-fraction while benign-transition-zone benign tissues exhibited hindered- and free-fractions, reflecting respective histological characteristics. Histologically-heterogeneous Grade 1 tumor consists of tumor/epithelial cells, stromal cells, and lumen structures. Modified-DBSI diffusion profiles include restricted-, hindered- and free-isotropic-diffusion-fractions consistent with this histological heterogeneity. Profiles of grades 2/3 PCa included restricted- and hindered-isotropic-diffusion signals with little to no free-diffusion signal for grade 3 but comparable extent of free-isotropic-diffusion signal, indicating an increased number of tumor/epithelial cells and a decrease in luminal structures of grade 3 tumors. Grades 4/5 tumors exhibited significant restricted-fraction with almost no signals from hindered- or free-isotropic-diffusion-fraction. Inflammation was more severe in grade 5 tumor. Profiles of grades 4/5 tumors reflecting the histological characteristics of sheets of tumor cells with complete absence of duct-acinar structures and stroma.
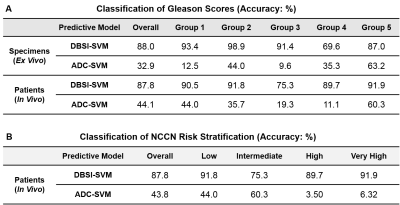
ADC/Modified-DBSI-SVM-based Gleason grading on prostatectomy and biopsied specimens compared with pathological findings revealing modified-DBSI-SVM achieved an 88.0% accuracy in overall Gleason score classification from ex vivo prostatectomy specimens and 87.8% accuracy for in vivo biopsied specimens. In contrast, ADC-SVM classification reported only 36.0% and 32.9% accuracy for ex vivo and in vivo respectively. Modified-DBSI-SVM also correctly categorized 87.9% of the cases into their NCCN cancer risk groups, while ADC-SVM afforded 41.1% accuracy (Table 1).
Discussion and Conclusion
We demonstrated modified-DBSI captured and quantified heterogeneous diffusion fingerprints reflecting prostatic histopathology, capable of noninvasively grading PCa. The diagnostic power of modified-DBSI could prevent low-grade cancer patients from undergoing unnecessary and costly invasive procedures, offering patients more reliable assessments on cancer progression during active surveillance, and helping patients and clinicians to determine the most appropriate treatment options. Accurate NCCN risk prediction by modified-DBSI could provide improved prognosis, which is especially helpful to patients undergoing active surveillance.Acknowledgements
This work was supported in part by NIH R01-NS047592, P01-NS059560, U01-EY025500, National Multiple Sclerosis Society (NMSS) RG 5258-A-5, RG 1701-26617, and Department of Defense Idea Award W81XWH-12-1-0457.References
1 Mertan, F. V. et al. Prospective Evaluation of the Prostate Imaging Reporting and Data System Version 2 for Prostate Cancer Detection. The Journal of urology 196, 690-696, doi:10.1016/j.juro.2016.04.057 (2016).
2 Yamin, G. et al. Voxel Level Radiologic-Pathologic Validation of Restriction Spectrum Imaging Cellularity Index with Gleason Grade in Prostate Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 22, 2668-2674, doi:10.1158/1078-0432.CCR-15-2429 (2016).
Figures