0093
Comprehensive hemodynamic assessment in non-sedated neonates with CHD using motion-robust 3D radial phase-contrast MR1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Physiology, University of Toronto, Toronto, ON, Canada, 3Medical Biophysics, University of Toronto, Toronto, ON, Canada, 4Heart Centre, The Hospital for Sick Children, Toronto, ON, Canada, 5Paediatrics, University of Toronto, Toronto, ON, Canada
Synopsis
The complex hemodynamics in congenital heart disease (CHD) are difficult to visualize and quantify in neonates and young infants. Here we present a novel motion robust and respiratory-resolved acquisition and reconstruction pipeline that addresses the need for rapid, high spatial resolution imaging in these patients. 3D cardiac flow is visualized and quantification comparison with 2D PC measurements is exhibited. This technique opens the door for more comprehensive investigations into the wealth of hemodynamic information not normally considered in surgical planning and follow-up evaluations of CHD.
Background:
Volumetric cardiovascular MR exams of neonates and young infants are technically challenging owing to the need for high spatial resolution and potential corruption through bulk and respiratory motion. These sources of motion are pronounced in young patients with complex congenital heart disease (CHD), who are preferably scanned in a feed-and-sleep manner to avoid sedation1,2. For assessment of complex cardiac hemodynamics using multidimensional phase contrast (3D PC), for example with 4D flow MRI3, motion effects can be further exacerbated due to long acquisitions. An alternative to conventional 3D Cartesian PC is 3D radial imaging using a double-golden-angle trajectory4,5. This provides multiple benefits: volumetric coverage with high isotropic spatial resolution, incoherent streaking artifacts from undersampling that can be mitigated using compressed sensing resulting in shorter scan times, and detection and compensation of bulk and respiratory motion through repeated sampling of the k-space center. The purpose of this study was the development of a framework for measuring and correcting motion using 3D radial MR with multi-dimensional flow sensitivity, and to implement this in neonates and young infants with CHD to investigate complex 3D blood flow patterns.Methods:
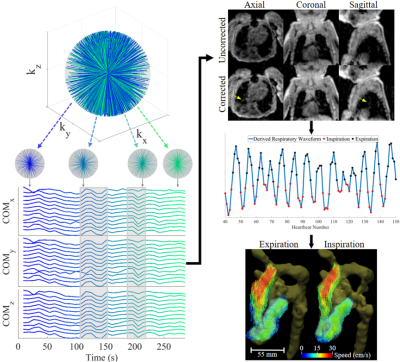
As part of an ongoing research study, 9 patients with CHD ranging from 10 to 151 days of age (Table 1) were imaged in axial orientation on a 1.5T scanner (MAGNETOM AvantoFIT, Siemens Healthcare, Erlangen, Germany). Acquisitions consisted of a free-running high isotropic resolution (0.60 – 0.94 mm) double-golden-angle 3D radial sequence5 with phase contrast velocity encoding applied along each spatial dimension. Figure 1 outlines the acquisition and reconstruction pipeline. Briefly, consecutive blocks of 1000 k-space projections are used to detect bulk motion6. The data are then divided into motion-free subsets and small subsets (<2000 projections) are discarded. The remaining motion-free subsets are reconstructed into low resolution images, which are co-registered using 3D rigid registration. Motion corrections (translations and rotations) are then applied to the original k-space. Inspiration and expiration phases are detected from heartbeat-averaged data, and velocity data is reconstructed in each of these phases using compressed sensing reconstruction with 3D spatial total variation (λ = 0.01). Due to high undersampling from short scan times, data are cardiac time-averaged for final reconstruction. Images were processed using Siemens 4D Flow v2.47. In both inspiration and expiration reconstructed datasets, time-averaged blood flows (normalized to body surface area) were quantified in individual great vessels. When available, these were validated against standard clinical 2D PC MRI (multiple averages without respiratory gating) using least squares regression.Results:
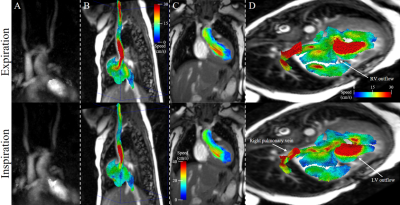
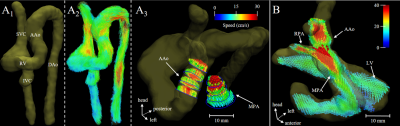
Acquisition, reconstruction, and processing were successfully completed in all subjects. Reconstruction time was approximately 15 minutes per patient. Qualitative comparisons of flow in two patients are presented in Figures 2 & 3. Visualizations between inspiration and expiration for a single patient are shown in Figure 2, displaying differences in both inflow and outflow blood speed throughout the volume. The single pre-operative patient is shown in Figure 3A, with example segmentation, whole volume streamlines, and velocity vector visualization in the parallel great arteries. Figure 3B shows anterior positioning of the distal and branch pulmonary arteries following the LeCompte maneuver in Patient 3. Quantitatively, 2D PC versus 3D radial flow yielded flow comparisons in 29 individual great vessels across all subjects, and demonstrated strong correlation (R2 = 0.94, slope = 0.95, p < 0.0001), while inspiration differed more from free-breathing 2D PC (R2 = 0.77, slope = 0.84, p < 0.0001). Across all patients and detected motion subsets, translations (standard deviation, [range]) in number of voxels were: left-right = 0.74 [0.03-4.19], anterior-posterior = 0.16 [0.03-1.13], and head-foot = 0.36 [0.04-2.52], while rotations in degrees (standard deviation, [range]) were very small at azimuthal = 0.38 [0-2.56] and polar = 0.39 [0-3.62].Discussion:
Here we present an advanced approach for hemodynamic assessment in a technically demanding clinical population. The ability of 3D radial acquisitions to resolve and correct for thoracic motion is demonstrated, providing a wealth of additional information in the form of respiratory-resolved blood flows over the entire neonatal chest. Good correlation of clinically obtained flows with the expiration phase lend confidence to the accuracy of the proposed technique.Conclusion:
This work demonstrates the feasibility of a fast, motion-robust volumetric velocity imaging technique performed in neonates and infants with CHD. Further investigation into respiratory induced differences may be clinically relevant for surgical planning or post-operative assessment. Future work will focus on the extension of this technique to acquire and resolve cardiac CINE imaging for dynamic visualization and assessment of these small structures with complex flow patterns.Acknowledgements
No acknowledgement found.References
1. Shariat M, et al. Utility of feed-and-sleep cardiovascular magnetic resonance in young infants with complex cardiovascular disease. Pediatr Cardiol 36, 809-812 (2015).
2. Antonov NK, et al. Feed and Wrap MRI Technique in Infants. Clin Pediatr (Phila) 56, 1095-1103 (2017).
3. Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging 36, 1015-1036 (2012).
4. Gu T, et al. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol 26, 743-749 (2005).
5. Chan RW, Ramsay EA, Cunningham CH, Plewes DB. Temporal stability of adaptive 3D radial MRI using multidimensional golden means. Magn Reson Med 61, 354-363 (2009).
6. Anderson AG, 3rd, Velikina J, Block W, Wieben O, Samsonov A. Adaptive retrospective correction of motion artifacts in cranial MRI with multicoil three-dimensional radial acquisitions. Magn Reson Med 69, 1094-1103 (2013).
7. Gulsun MA, et al. A novel 4D flow tool for comprehensive blood flow analysis. In: International Society for Magnetic Resonance in Medicine, Melbourne, AUS (2012).
Figures
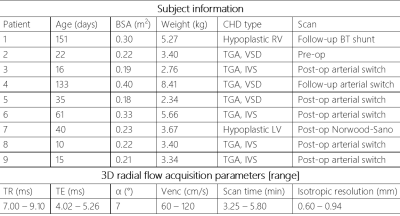
Table 1. Patient information and acquisition parameters.
RV – right ventricle; TGA – transposition of the great arteries; VSD – ventricular septal defect; IVS – intact ventricular septum; LV – left ventricle