0047
Dynamic Deuterium MRS Imaging for Studying Rat Heart Energy Metabolism in vivo – Initial ExperienceHuan Li1,2, Xiao-Hong Zhu1, Wei Zhu1, Byeong-Yeul Lee1, Hannes Michel Wiesner1, Yi Zhang1, Tao Wang1, and Wei Chen1
1Center for Magnetic Resonance Research,Department of Radiology, University of Minnesota, Minneapolis, MN, United States, Minneapolis, MN, United States, 2Department of Radiology, Zhongnan Hospital of Wuhan University, Wuhan University, Wuhan, China
Synopsis
Assessment of myocardial energy metabolism is crucial for understanding heart function and viable myocardium after myocardial infarction. Based on our recently developed in vivo Deuterium (2H) MR spectroscopic (DMRS) approach, we further exploited the DMRS and imaging (DMRSI) methods for dynamic measurement of the myocardial energy metabolism in rat heart at 16.4 T. This work demonstrates the feasibility of in vivo DMRS for assessing myocardial energy metabolisms, and its potential to directly image the viable myocardium in hearts under conditions such as myocardial infarction.
Introduction
Assessment of energy metabolism is crucial for understanding myocardial activity and function under physiopathological conditions. Myocardium mainly obtained energy via aerobic oxidation of various energy substrates including fatty acids (>60%) and glucose. MR perfusion imaging and SPECT perfusion imaging are commonly used to assess blood supply of the myocardium, myocardial ischemia and viability of the myocardium (1). However, these methods are unable to evaluate the myocardial cellular activity and metabolism directly. Recently, we developed a novel Deuterium (2H) MR (DMR) spectroscopic (DMRS) approach for assessing energy metabolisms in preclinical rat brain at ultrahigh field (2). This new method is advantageous since it eliminates the radioactive tracer commonly employed in SPECT imaging of myocardial metabolism; and it allows more signal averaging to gain sensitivity per unit time owing to shorter T1 of deuterated metabolites with quadrupolar relaxation mechanism. In this study, we aimed to develop and utilize the dynamic DMRS method for assessing myocardial energy metabolisms in the rat heart at 16.4 T.Method
Three Sprague Dawley rats (BW=432±34 g) were anesthetized by 2% isoflurane, and their femoral arteries and veins were catheterized for blood sampling, physiological monitoring and deuterated glucose and/or acetate infusion. Rectal temperature was maintained at 37±1℃ using heated circulating water. The animal study protocol was approved by the Institutional Animal Care and Use Committee of University of Minnesota, and were compliance with the ARRIVE guidelines. All MR experiments were conducted at 16.4 T/26 cm bore scanner (Varian/VNMRJ) using a small (~1.2cm diameter) 2H surface coil for deuterium data acquisition and a large (~3.5cm diameter) 1H surface coil for anatomical imaging. The 2H coil was inserted into the rat chest via thoracotomy and placed between the chest wall and the heart with the animal in prone position. The 1H surface coil was placed at the left side of the chest at the same level of the heart (see Figure 1). A single-pulse-acquire sequence was applied to obtain dynamic DMR spectra with 30 s temporal resolution for 60 min. For each rat, 2 min baseline spectra were acquired followed by 5 min i.v. infusion of deuterated D-Glucose-6,6-d (d66, Cambridge Isotope Laboratories, Inc., 1.0 g/kg BW) or deuterated sodium acetate-d3 (Sigma Aldrich, 1.0 g/kg BW) dissolved in 2.5 mL saline. The 2H 3D-CSI data of rat hearts were also acquired before and after injection of glucose and/or acetate with 124 mL nominal spatial resolution in approximately 5 min (spectral width=3 kHz; TR = 45 ms; FOV=4*4*4 cm3, 9*9*5 phase encodes, 15 averages). All resonance signals (deuterated water, glucose (Glc), glutamate/glutamine (Glx), acetate) were fitted using a MATLAB based program (2).Result
We were able to successfully place the 2H surface coil underneath the heart and reduce the heart motion caused by breathing during the experiment; the position of the heart and coils were illustrated in Figure 1; it also displays DMRS imaging of natural abundance Deuterium-labeled water with excellent sensitivity and spectral quality in rat heart in vivo. Figure 2 shows representative DMR spectra of rat hearts (black and red traces represent original and fitted spectra) under 4 min post-infusion of deuteriated-acetate (a) and 10 min post-infusion of deuteriated-glucose (b). Model-decomposed individual components of deuterated water, acetate, glucose and Glx could be characterized clearly. Figure 3a displays the time courses of deuterated glucose, Glx and water signals before, during and after infusion of d66 based on the model fitting of the in vivo 2H spectra as shown in Figure 2a. Figure 3b showed the time courses of deuterated water with glucose and acetate infusion, respectively. Significantly higher production of the deuterated water was observed with acetate infusion as compared to the glucose infusion.Discussion & Conclusion
Myocardium mainly produces ATP energy via aerobic oxidation of various substrates including fatty acids and glucose with preference on fatty acids. The observed higher deuteriated-water production under acetate infusion in the heart could be due to the higher concentration of 2H in acetate infusion than that of glucose; 1g /kg BW of acetate and glucose was used for this study, the concentration of 2H in acetate is about 3.2 times higher than that of glucose. This work demonstrates the feasibility and sensitivity of the in vivo DMR imaging technique for assessing myocardial energy metabolisms, which makes it possible to image the energy metabolism and assess cardiomyocyte activity in hearts under normal and diseased states (such as myocardial ischemia&myocardial infarction) at high/ultrahigh field. Further studies and quantification modeling are needed to find more characteristics of cardiac energy metabolism.Acknowledgements
NIH Grants: R01 NS057560, NS070839, MH111447, R24 MH106049, U01EB026978, P41 EB015894, P30 NS076408, S10 RR025031.References
(1) Richard A.P. Takx, et al. (2015) Circ Cardiovasc Imaging. 8(1), e002666
(2) Lu, M. et al. (2017) J Creb Blood Flow Metab.17(11), 3518-3530
Figures
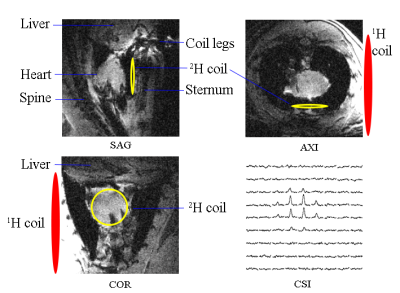
Figure 1. Anatomical images and DMR-CSI
slice extracted from 3D data of a representative rat heart. The heart 2H
coil was inserted into the chest and placed onto the surface of heart. The 1H
surface coil was placed at the left side of the chest at the same level of the
heart.
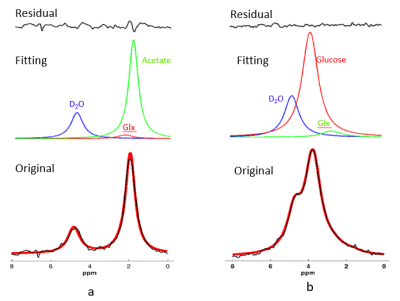
Figure 2. Representative original (black
trace in bottom row) and fitted (red trace in bottom row) DMR spectra obtained
from a rat heart under post-deuterated acetate infusion 4 min later (a) or
glucose infusion 10 min later (b). Model-decomposed individual components of deuterated
water, acetate, glucose and Glx were showed in the second row and small
fitting residue was displayed at the top row. Each in vivo spectrum displayed in this
figure was summed from 30s of data acquisitions. 2H resonance
assignments: (1) water (4.8 ppm, set as a chemical shift reference); (2) acetate
(1.8 ppm); (3) glucose (3.8 ppm), (4) Glx (2.4 ppm).
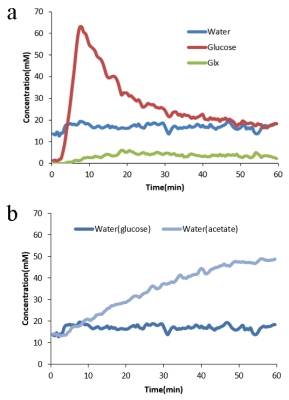
Figure 3. Figure 3a showed the time courses
of deuterated glucose, Glx and water signals before and after glucose infusion
based on the original in vivo 2H spectra. Figure 3b showed the time
courses of deuterated water before and after glucose or acetate infusion. Baseline spectras were acquired for 2 min followed by 5 min i.v. infusion of
deuterated glucose or deuterated acetate (1.0 g/kg BW) with 30 s temporal
resolution and total scan time was 60 min.