0044
The Relation Between Nasal Nitric Oxide and Neurocognitive Outcome is Mediated by Regional Cerebral Blood Flow in Healthy Adolescents and Adolescents with Congenital Heart Disease1Radiology, UPMC Pittsburgh Children's Hospital, Pittsburgh, PA, United States, 2UPMC Pittsburgh Children's Hospital, Pittsburgh, PA, United States
Synopsis
Nasal nitric oxide (nNO) may be a proxy for NO or NO synthase (NOS) availability in the brain, while NO/NOS availability is likely related to metabolism and downstream to neurocognitive function. We tested this hypothesis using a mediation analysis with nNO the independent variable, regional CBF (rCBF) as measured by ASL the mediator, and neurocognitive outcome (NIH Toolbox) the dependent variable in a cohort of normal adolescents and adolescents with congenital heart disease. Anterior and posterior default mode network regions positively mediated all NIH Toolbox composite scores, especially crystallized cognition. Results indicate nNO may be a powerful biomarker for brain function and metabolism.
Introduction
Nitric oxide (NO) is known to be a mediator of vasodilation following neuronal activity and thus an important component of neurovascular unit (NVU) function. However, non-invasive in vivo measurement of cerebral NO is infeasible. A possible proxy for cerebral NO is nasal nitric oxide (nNO). shown to be a biomarker for poor cardiac function in individuals with congenital heart disease (CHD) [1], and previously shown to be related to regional cerebral blood flow (rCBF) using ASL [2]. We investigate the relation of nNO to neurocognitive outcome in normal healthy adolescents and adolescents with CHD via performing a mediation analysis with rCBF as the mediator.Materials and Methods
Participants: 22 healthy controls and 17 CHD patients were scanned. No significant difference between the groups were seen on any of the demographic or neurocognitive variables with the exception of the Picture Sequence Memory subtest (Figure 1).
Neurocognitive Assessment: Participants were administered the NIH Toolbox cognitive battery. Seven individual tests were performed, and age-corrected composite scores including total cognition, fluid cognition, and crystallized cognition were computed.
MRI Scans: Scans were acquired on Siemens 3T Skyra system. Pseudo-continuous ASL (PCASL) was obtained using a 2D GE-EPI readout. Parameters: Labeling duration = 1500 ms, post-label delay = 1200 ms, TR = 4000 ms, 45 label/control pairs acquired, 2-D EPI acquisition, TE = 12 ms, resolution = 4 X 4 X 5 mm.
nNO Measurements: nNO values were acquired using tidal breath sampling with a CLD 88sp NO analyzer obtained via low continuous suction at a rate of 0.3 L/min from each naris using a nasal olive sampling cannula during tidal breathing. Each naris was sampled once for 50 s. Five peak inflections from nNO concentration curve were analyzed against the sampling flow and averaged to yield a final value in nl/min.
ASL Pre-Processing: Images were motion-corrected, and fractional change images computed using a voxelwise GLM, with motion and drift parameters included as nuisance covariates. Using the two-compartment model [3], rCBF maps were computed. The reference EPI images were segmented into gray matter images using routines in SPM8 and transformed into MNI space using a study-specific gray matter template. The rCBF map was spatially normalized into MNI space using the same transformation.
Mediation: Mediation analyses [4] were performed on a voxelwise basis with nNO the independent variable, neurocognitive outcome the independent variable, and rCBF the mediator. Age at scan, age at nNO measurement, sex, voxelwise gray matter probability, and rms motion during the pCASL scan were included as covariates. Statistical significance was performed using bootstrapping (1000 repetitions) with bias-corrected and accelerated confidence intervals [5]. Results were deemed significant at FWE-corrected p < 0.05, determined via Monte Carlo analysis [6].
Results and Discussion
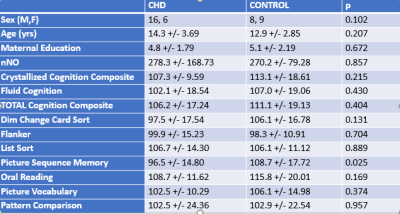
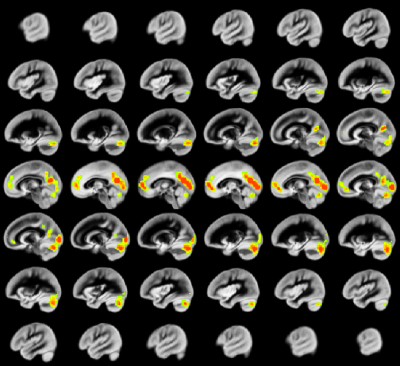
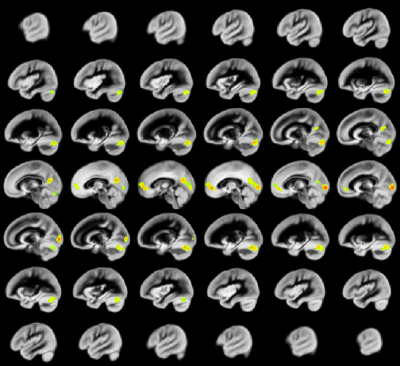
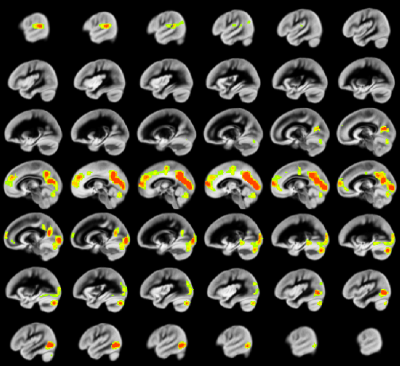
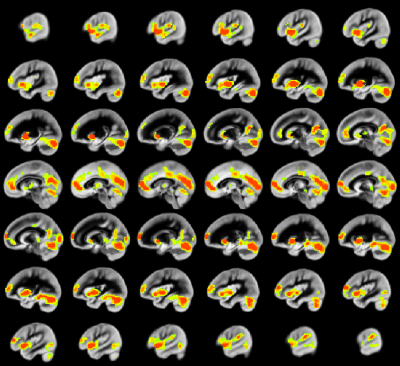
A relation between nNO and total cognition composite score is positively mediated via rCBF chiefly in posterior and anterior default mode network regions (DMN; Figure 2). These regions are also implicated in mediation of fluid cognition (Figure 3) and especially crystallized cognition (Figure 4) in addition to lateral temporal-occipital regions. For the Picture Sequence memory subtest (Figure 5), the relation is also mediated by rCBF in salience network regions (insula, medial frontal).
As rCBF is highly correlated with resting-state metabolism, our results are consistent with hypometabolism and NVU dysfunction from decreased NO synthase (NOS) availability during development which results in adverse neurocognitive outcome. Lack of NO/NOS availability is likely to especially affect regions with high metabolism such as the DMN, and aberrant resting-state DMN function and metabolism will adversely affect neurocognitive function.
Our results also show the usefulness of nNO as a proxy for NO/NOS availability. However, the precise relationship between nNO and cerebral NO is as yet unknown. There are three known isoforms of NOS [7]: neuronal, endothelial, and inducible. We would hypothesize a genetic etiology responsible for low levels of all isoforms of NOS which also may be related to CHD and other pathologies such as primary ciliary dyskinesia.
Further research will also elucidate the precise relationship with CHD. We have previously shown that reduced rCBF primarily in subcortical and salience network regions mediates adverse neurocognitive outcome in CHD [8]; whether this is a separate effect from that seen from low nNO or related to it is unknown.
Conclusion
Neurocognitive outcome is associated with nNO and mediated via regional CBF in normal adolescents and adolescents with CHD. Results suggest NO/NOS availability may be essential for optimal metabolism and neurocognitive function and that nNO may be a useful biomarker.Acknowledgements
No acknowledgement found.References
[1] Adams PS et al. J Am Heart Assoc 2017; 6(12): e007447.
[2] Schmithorst VJ et al. Proc ISMRM 2018; 850.
[3] Alsop DC, Detre JA. J Cereb Blood Flow Metab 1996; 16: 1236-49.
[4] Hayes AF, Scharkow M. Psychol Sci 2013; 24: 1218-27.
[5] DiCiccio T, Efron B. Statistical Science 1996; 13: 189-228.
[6] Ledberg A et al. Neuroimage 1998; 8: 113-28.
[7] Forstermann U, Sessa WC. Eur Heart J 2012; 33(7): 829-837.
[8] Schmithorst VJ et al. Proc ISMRM 2018; 1791.
Figures